Lysine11-Linked Polyubiquitination of the AnkB F-Box Effector of Legionella pneumophila
- PMID: 26483404
- PMCID: PMC4694019
- DOI: 10.1128/IAI.01165-15
Lysine11-Linked Polyubiquitination of the AnkB F-Box Effector of Legionella pneumophila
Abstract
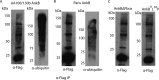
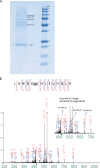
The fate of the polyubiquitinated protein is determined by the lysine linkages involved in the polymerization of the ubiquitin monomers, which has seven lysine residues (K(6), K(11), K(27), K(29), K(33), K(48), and K(63)). The translocated AnkB effector of the intravacuolar pathogen Legionella pneumophila is a bona fide F-box protein, which is localized to the cytosolic side of the Legionella-containing vacuole (LCV) and is essential for intravacuolar proliferation within macrophages and amoebae. The F-box domain of AnkB interacts with the host SCF1 E3 ubiquitin ligase that triggers the decoration of the LCV with K(48)-linked polyubiquitinated proteins that are targeted for proteasomal degradation. Here we report that AnkB becomes rapidly polyubiquitinated within the host cell, and this modification is independent of the F-box domain of AnkB, indicating host-mediated polyubiquitination. We show that the AnkB effector interacts specifically with the host E3 ubiquitin ligase Trim21. Mass spectrometry analyses have shown that AnkB is modified by K(11)-linked polyubiquitination, which has no effect on its stability. This work shows the first example of K(11)-linked polyubiquitination of a bacterial effector and its interaction with the host Trim21 ubiquitin ligase.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
Molecular mimicry by an F-box effector of Legionella pneumophila hijacks a conserved polyubiquitination machinery within macrophages and protozoa.PLoS Pathog. 2009 Dec;5(12):e1000704. doi: 10.1371/journal.ppat.1000704. Epub 2009 Dec 24. PLoS Pathog. 2009. PMID: 20041211 Free PMC article.
-
Indispensable role for the eukaryotic-like ankyrin domains of the ankyrin B effector of Legionella pneumophila within macrophages and amoebae.Infect Immun. 2010 May;78(5):2079-88. doi: 10.1128/IAI.01450-09. Epub 2010 Mar 1. Infect Immun. 2010. PMID: 20194593 Free PMC article.
-
The Legionella pneumophila F-box protein Lpp2082 (AnkB) modulates ubiquitination of the host protein parvin B and promotes intracellular replication.Cell Microbiol. 2010 Sep 1;12(9):1272-91. doi: 10.1111/j.1462-5822.2010.01467.x. Epub 2010 Mar 25. Cell Microbiol. 2010. PMID: 20345489
-
Evolution and Adaptation of Legionella pneumophila to Manipulate the Ubiquitination Machinery of Its Amoebae and Mammalian Hosts.Biomolecules. 2021 Jan 15;11(1):112. doi: 10.3390/biom11010112. Biomolecules. 2021. PMID: 33467718 Free PMC article. Review.
-
Amoeba host-Legionella synchronization of amino acid auxotrophy and its role in bacterial adaptation and pathogenic evolution.Environ Microbiol. 2014 Feb;16(2):350-8. doi: 10.1111/1462-2920.12290. Epub 2013 Oct 21. Environ Microbiol. 2014. PMID: 24112119 Free PMC article. Review.
Cited by
-
Ubiquitin-targeted bacterial effectors: rule breakers of the ubiquitin system.EMBO J. 2023 Sep 18;42(18):e114318. doi: 10.15252/embj.2023114318. Epub 2023 Aug 9. EMBO J. 2023. PMID: 37555693 Free PMC article. Review.
-
The ubiquitin system: a critical regulator of innate immunity and pathogen-host interactions.Cell Mol Immunol. 2016 Sep;13(5):560-76. doi: 10.1038/cmi.2016.40. Epub 2016 Aug 15. Cell Mol Immunol. 2016. PMID: 27524111 Free PMC article. Review.
-
Divergent evolution of Di-lysine ER retention vs. farnesylation motif-mediated anchoring of the AnkB virulence effector to the Legionella-containing vacuolar membrane.Sci Rep. 2017 Jul 11;7(1):5123. doi: 10.1038/s41598-017-05211-5. Sci Rep. 2017. PMID: 28698607 Free PMC article.
-
Global atlas of predicted functional domains in Legionella pneumophila Dot/Icm translocated effectors.Mol Syst Biol. 2025 Jan;21(1):59-89. doi: 10.1038/s44320-024-00076-z. Epub 2024 Nov 19. Mol Syst Biol. 2025. PMID: 39562741 Free PMC article.
-
Structural Mimicry by a Bacterial F Box Effector Hijacks the Host Ubiquitin-Proteasome System.Structure. 2017 Feb 7;25(2):376-383. doi: 10.1016/j.str.2016.12.015. Epub 2017 Jan 19. Structure. 2017. PMID: 28111017 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

