Treatment with Interleukin-7 Restores Host Defense against Pneumocystis in CD4+ T-Lymphocyte-Depleted Mice
- PMID: 26483405
- PMCID: PMC4694009
- DOI: 10.1128/IAI.01189-15
Treatment with Interleukin-7 Restores Host Defense against Pneumocystis in CD4+ T-Lymphocyte-Depleted Mice
Abstract
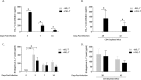
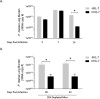
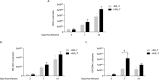
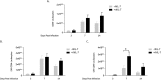
Pneumocystis pneumonia (PCP) is a major cause of morbidity and mortality in patients with HIV infection. CD4(+) T lymphocytes are critical for host defense against this infection, but in the absence of CD4(+) T lymphocytes, CD8(+) T lymphocytes may provide limited host defense. The cytokine interleukin-7 (IL-7) functions to enhance lymphocyte proliferation, survival, and recruitment of immune cells to sites of infection. However, there is little known about the role of IL-7 in PCP or its potential use as an immunotherapeutic agent. We hypothesized that treatment with recombinant human IL-7 (rhIL-7) would augment host defense against Pneumocystis and accelerate pathogen clearance in CD4-depleted mice. Control and CD4-depleted mice were infected with Pneumocystis, and rhIL-7 was administered via intraperitoneal injection. Our studies indicate that endogenous murine IL-7 is part of the normal host response to Pneumocystis murina and that administration of rhIL-7 markedly enhanced clearance of Pneumocystis in CD4-depleted mice. Additionally, we observed increased recruitment of CD8(+) T lymphocytes to the lungs and decreased apoptosis of pulmonary CD8(+) T lymphocytes in rhIL-7-treated animals compared to those in untreated mice. The antiapoptotic effect of rhIL-7 was associated with increased levels of Bcl-2 protein in T lymphocytes. rhIL-7 immunotherapy in CD4-depleted mice also increased the number of gamma interferon (IFN-γ)-positive CD8(+) central memory T lymphocytes in the lungs. We conclude that rhIL-7 has a potent therapeutic effect in the treatment of murine Pneumocystis pneumonia in CD4-depleted mice. This therapeutic effect is mediated through enhanced recruitment of CD8(+) T cells and decreased apoptosis of lung T lymphocytes, with a preferential action on central memory CD8(+) T lymphocytes.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
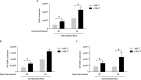
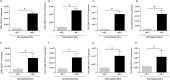

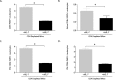

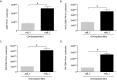
References
-
- Masur H, Michelis MA, Greene JB, Onorato I, Stouwe RA, Holzman RS, Wormser G, Brettman L, Lange M, Murray HW, Cunningham-Rundles S. 1981. An outbreak of community-acquired Pneumocystis carinii pneumonia: initial manifestation of cellular immune dysfunction. N Engl J Med 305:1431–1438. doi:10.1056/NEJM198112103052402. - DOI - PubMed
-
- Kolls JK, Habetz S, Shean MK, Vazquez C, Brown JA, Lei D, Schwarzenberger P, Ye P, Nelson S, Summer WR, Shellito JE. 1999. IFN-gamma and CD8+ T cells restore host defenses against Pneumocystis carinii in mice depleted of CD4+ T cells. J Immunol 162:2890–2894. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

