Activation of the Classical Mitogen-Activated Protein Kinases Is Part of the Shiga Toxin-Induced Ribotoxic Stress Response and May Contribute to Shiga Toxin-Induced Inflammation
- PMID: 26483408
- PMCID: PMC4694011
- DOI: 10.1128/IAI.00977-15
Activation of the Classical Mitogen-Activated Protein Kinases Is Part of the Shiga Toxin-Induced Ribotoxic Stress Response and May Contribute to Shiga Toxin-Induced Inflammation
Erratum in
-
Correction for Jandhyala et al., "Activation of the Classical Mitogen-Activated Protein Kinases Is Part of the Shiga Toxin-Induced Ribotoxic Stress Response and May Contribute to Shiga Toxin-Induced Inflammation".Infect Immun. 2017 Dec 19;86(1):e00753-17. doi: 10.1128/IAI.00753-17. Print 2018 Jan. Infect Immun. 2017. PMID: 29259142 Free PMC article. No abstract available.
Abstract
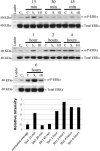
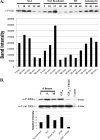
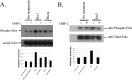
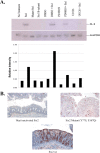
Infection with enterohemorrhagic Escherichia coli (EHEC) can result in severe disease, including hemorrhagic colitis and the hemolytic uremic syndrome. Shiga toxins (Stx) are the key EHEC virulence determinant contributing to severe disease. Despite inhibiting protein synthesis, Shiga toxins paradoxically induce the expression of proinflammatory cytokines from various cell types in vitro, including intestinal epithelial cells (IECs). This effect is mediated in large part by the ribotoxic stress response (RSR). The Shiga toxin-induced RSR is known to involve the activation of the stress-activated protein kinases (SAPKs) p38 and JNK. In some cell types, Stx also can induce the classical mitogen-activated protein kinases (MAPKs) or ERK1/2, but the mechanism(s) by which this activation occurs is unknown. In this study, we investigated the mechanism by which Stx activates ERK1/2s in IECs and the contribution of ERK1/2 activation to interleukin-8 (IL-8) expression. We demonstrate that Stx1 activates ERK1/2 in a biphasic manner: the first phase occurs in response to StxB1 subunit, while the second phase requires StxA1 subunit activity. We show that the A subunit-dependent ERK1/2 activation is mediated through ZAK-dependent signaling, and inhibition of ERK1/2 activation via the MEK1/2 inhibitors U0126 and PD98059 results in decreased Stx1-mediated IL-8 mRNA. Finally, we demonstrate that ERK1/2 are activated in vivo in the colon of Stx2-intoxicated infant rabbits, a model in which Stx2 induces a primarily neutrophilic inflammatory response. Together, our data support a role for ERK1/2 activation in the development of Stx-mediated intestinal inflammation.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
ZAK: a MAP3Kinase that transduces Shiga toxin- and ricin-induced proinflammatory cytokine expression.Cell Microbiol. 2008 Jul;10(7):1468-77. doi: 10.1111/j.1462-5822.2008.01139.x. Epub 2008 Mar 10. Cell Microbiol. 2008. PMID: 18331592
-
Shiga toxin 1 triggers a ribotoxic stress response leading to p38 and JNK activation and induction of apoptosis in intestinal epithelial cells.Infect Immun. 2003 Mar;71(3):1497-504. doi: 10.1128/IAI.71.3.1497-1504.2003. Infect Immun. 2003. PMID: 12595468 Free PMC article.
-
Shiga toxin 2 and flagellin from shiga-toxigenic Escherichia coli superinduce interleukin-8 through synergistic effects on host stress-activated protein kinase activation.Infect Immun. 2010 Jul;78(7):2984-94. doi: 10.1128/IAI.00383-10. Epub 2010 May 3. Infect Immun. 2010. PMID: 20439475 Free PMC article.
-
Shiga toxin-glycosphingolipid interaction: Status quo of research with focus on primary human brain and kidney endothelial cells.Int J Med Microbiol. 2018 Dec;308(8):1073-1084. doi: 10.1016/j.ijmm.2018.09.003. Epub 2018 Sep 8. Int J Med Microbiol. 2018. PMID: 30224239 Review.
-
Shiga toxin interaction with human intestinal epithelium.Toxins (Basel). 2011 Jun;3(6):626-39. doi: 10.3390/toxins3060626. Epub 2011 Jun 14. Toxins (Basel). 2011. PMID: 22069729 Free PMC article. Review.
Cited by
-
Shiga Toxins as Antitumor Tools.Toxins (Basel). 2021 Sep 28;13(10):690. doi: 10.3390/toxins13100690. Toxins (Basel). 2021. PMID: 34678982 Free PMC article. Review.
-
CRISPR Screen Reveals that EHEC's T3SS and Shiga Toxin Rely on Shared Host Factors for Infection.mBio. 2018 Jun 19;9(3):e01003-18. doi: 10.1128/mBio.01003-18. mBio. 2018. PMID: 29921669 Free PMC article.
-
Role of Recent Therapeutic Applications and the Infection Strategies of Shiga Toxin-Producing Escherichia coli.Front Cell Infect Microbiol. 2021 Jun 29;11:614963. doi: 10.3389/fcimb.2021.614963. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34268129 Free PMC article. Review.
-
Valid Presumption of Shiga Toxin-Mediated Damage of Developing Erythrocytes in EHEC-Associated Hemolytic Uremic Syndrome.Toxins (Basel). 2020 Jun 4;12(6):373. doi: 10.3390/toxins12060373. Toxins (Basel). 2020. PMID: 32512916 Free PMC article. Review.
-
Early Response to the Plant Toxin Stenodactylin in Acute Myeloid Leukemia Cells Involves Inflammatory and Apoptotic Signaling.Front Pharmacol. 2020 May 8;11:630. doi: 10.3389/fphar.2020.00630. eCollection 2020. Front Pharmacol. 2020. PMID: 32457623 Free PMC article.
References
-
- Tarr PI, Gordon CA, Chandler WL. 2005. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 365:1073–1086. - PubMed
-
- Vanaja SK, Jandhyala DM, Mallick EM, Leong JM, Balasubramanian S. 2013. Enterohemorrhagic and other Shiga-toxin-producing Escherichia coli. In Donnenberg MS. (ed), Escherichia coli, pathotypes and principles of pathogenesis, 2nd ed Elsevier, Boston, MA.
-
- Iordanov MS, Pribnow D, Magun JL, Dinh TH, Pearson JA, Chen SL, Magun BE. 1997. Ribotoxic stress response: activation of the stress-activated protein kinase JNK1 by inhibitors of the peptidyl transferase reaction and by sequence-specific RNA damage to the alpha-sarcin/ricin loop in the 28S rRNA. Mol Cell Biol 17:3373–3381. doi:10.1128/MCB.17.6.3373. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

