Morphology-Independent Virulence of Candida Species during Polymicrobial Intra-abdominal Infections with Staphylococcus aureus
- PMID: 26483410
- PMCID: PMC4694008
- DOI: 10.1128/IAI.01059-15
Morphology-Independent Virulence of Candida Species during Polymicrobial Intra-abdominal Infections with Staphylococcus aureus
Abstract
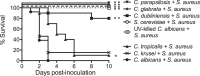
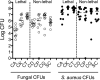
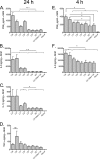
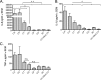
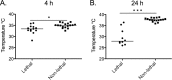
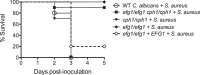
Intra-abdominal polymicrobial infections cause significant morbidity and mortality. An experimental mouse model of Candida albicans-Staphylococcus aureus intra-abdominal infection (IAI) results in 100% mortality by 48 to 72 h postinoculation, while monomicrobial infections are avirulent. Mortality is associated with robust local and systemic inflammation without a requirement for C. albicans morphogenesis. However, the contribution of virulence factors coregulated during the yeast-to-hypha transition is unknown. This also raised the question of whether other Candida species that are unable to form hyphae are as virulent as C. albicans during polymicrobial IAI. Therefore, the purpose of this study was to evaluate the ability of non-albicans Candida (NAC) species with various morphologies and C. albicans transcription factor mutants (efg1/efg1 and cph1/cph1) to induce synergistic mortality and the accompanying inflammation. Results showed that S. aureus coinoculated with C. krusei or C. tropicalis was highly lethal, similar to C. albicans, while S. aureus-C. dubliniensis, S. aureus-C. parapsilosis, and S. aureus-C. glabrata coinoculations resulted in little to no mortality. Local and systemic interleukin-6 (IL-6) and prostaglandin E2 (PGE2) levels were significantly elevated during symptomatic and/or lethal coinfections, and hypothermia strongly correlated with mortality. Coinoculation with C. albicans strains deficient in the transcription factor Efg1 but not Cph1 reversed the lethal outcome. These results support previous findings and demonstrate that select Candida species, without reference to any morphological requirement, induce synergistic mortality, with IL-6 and PGE2 acting as key inflammatory factors. Mechanistically, signaling pathways controlled by Efg1 are critical for the ability of C. albicans to induce mortality from an intra-abdominal polymicrobial infection.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- Heemken R, Gandawidjaja L, Hau T. 1997. Peritonitis: pathophysiology and local defense mechanisms. Hepatogastroenterology 44:927–936. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

