Rosuvastatin Alters the Proteome of High Density Lipoproteins: Generation of alpha-1-antitrypsin Enriched Particles with Anti-inflammatory Properties
- PMID: 26483418
- PMCID: PMC4762624
- DOI: 10.1074/mcp.M115.054031
Rosuvastatin Alters the Proteome of High Density Lipoproteins: Generation of alpha-1-antitrypsin Enriched Particles with Anti-inflammatory Properties
Abstract
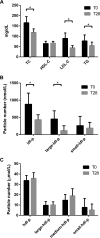
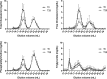
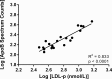
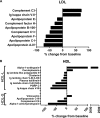
Statins lower plasma cholesterol by as much as 50%, thus reducing future cardiovascular events. However, the physiological effects of statins are diverse and not all are related to low density lipoprotein cholesterol (LDL-C) lowering. We performed a small clinical pilot study to assess the impact of statins on lipoprotein-associated proteins in healthy individuals (n = 10) with normal LDL-C (<130 mg/dL), who were treated with rosuvastatin (20 mg/day) for 28 days. Proteomic analysis of size-exclusion chromatography isolated LDL, large high density lipoprotein (HDL-L), and small HDL (HDL-S) fractions and spectral counting was used to compare relative protein detection before and after statin therapy. Significant protein changes were found in each lipoprotein pool and included both increases and decreases in several proteins involved in lipoprotein metabolism, complement regulation and acute phase response. The most dramatic effect of the rosuvastatin treatment was an increase in α-1-antirypsin (A1AT) spectral counts associated with HDL-L particles. Quantitative measurement by ELISA confirmed an average 5.7-fold increase in HDL-L associated A1AT. Molecular modeling predictions indicated that the hydrophobic reactive center loop of A1AT, the functional domain responsible for its protease inhibitor activity, is likely involved in lipid binding and association with HDL was found to protect A1AT against oxidative inactivation. Cell culture experiments, using J774 macrophages, demonstrated that the association of A1AT with HDL enhances its antiprotease activity, preventing elastase induced production of tumor necrosis factor α. In conclusion, we show that statins can significantly alter the protein composition of both LDL and HDL and our studies reveal a novel functional relationship between A1AT and HDL. The up-regulation of A1AT on HDL enhances its anti-inflammatory functionality, which may contribute to the non-lipid lowering beneficial effects of statins.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Kannel W. B., Castelli W. P., and Gordon T. (1979) Cholesterol in the prediction of atherosclerotic disease. New perspectives based on the Framingham study. Ann. Intern. Med. 90, 85–91 - PubMed
-
- Baigent C., Keech A., Kearney P. M., Blackwell L., Buck G., Pollicino C., Kirby A., Sourjina T., Peto R., Collins R., and Simes R. (2005) Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 366, 1267–1278 - PubMed
-
- Jain M. K., and Ridker P. M. (2005) Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat. Rev. Drug. Discov. 4, 977–987 - PubMed
-
- Chapman M. J., Guerin M., and Bruckert E. (1998) Atherogenic, dense low-density lipoproteins. Pathophysiology and new therapeutic approaches. Eur. Heart J. 19, A24–30 - PubMed
-
- Vaisar T., Pennathur S., Green P. S., Gharib S. A., Hoofnagle A. N., Cheung M. C., Byun J., Vuletic S., Kassim S., Singh P., Chea H., Knopp R. H., Brunzell J., Geary R., Chait A., Zhao X. Q., Elkon K., Marcovina S., Ridker P., Oram J. F., and Heinecke J. W. (2007) Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J. Clin. Invest. 117, 746–756 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

