Dystrophin-glycoprotein complex regulates muscle nitric oxide production through mechanoregulation of AMPK signaling
- PMID: 26483453
- PMCID: PMC4640723
- DOI: 10.1073/pnas.1512991112
Dystrophin-glycoprotein complex regulates muscle nitric oxide production through mechanoregulation of AMPK signaling
Abstract
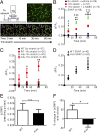
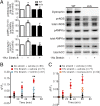
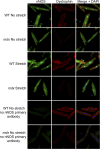
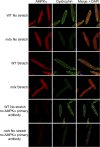
Patients deficient in dystrophin, a protein that links the cytoskeleton to the extracellular matrix via the dystrophin-glycoprotein complex (DGC), exhibit muscular dystrophy, cardiomyopathy, and impaired muscle nitric oxide (NO) production. We used live-cell NO imaging and in vitro cyclic stretch of isolated adult mouse cardiomyocytes as a model system to investigate if and how the DGC directly regulates the mechanical activation of muscle NO signaling. Acute activation of NO synthesis by mechanical stretch was impaired in dystrophin-deficient mdx cardiomyocytes, accompanied by loss of stretch-induced neuronal NO synthase (nNOS) S1412 phosphorylation. Intriguingly, stretch induced the acute activation of AMP-activated protein kinase (AMPK) in normal cardiomyocytes but not in mdx cardiomyocytes, and specific inhibition of AMPK was sufficient to attenuate mechanoactivation of NO production. Therefore, we tested whether direct pharmacologic activation of AMPK could bypass defective mechanical signaling to restore nNOS activity in dystrophin-deficient cardiomyocytes. Indeed, activation of AMPK with 5-aminoimidazole-4-carboxamide riboside or salicylate increased nNOS S1412 phosphorylation and was sufficient to enhance NO production in mdx cardiomyocytes. We conclude that the DGC promotes the mechanical activation of cardiac nNOS by acting as a mechanosensor to regulate AMPK activity, and that pharmacologic AMPK activation may be a suitable therapeutic strategy for restoring nNOS activity in dystrophin-deficient hearts and muscle.
Keywords: AMPK; cardiomyocyte; dystrophin; nNOS; stretch.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: The protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51(6):919–928. - PubMed
-
- Emery AE. Population frequencies of inherited neuromuscular diseases: A world survey. Neuromuscul Disord. 1991;1(1):19–29. - PubMed
-
- Campbell KP, Kahl SD. Association of dystrophin and an integral membrane glycoprotein. Nature. 1989;338(6212):259–262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

