The future of airborne sulfur-containing particles in the absence of fossil fuel sulfur dioxide emissions
- PMID: 26483454
- PMCID: PMC4640726
- DOI: 10.1073/pnas.1510743112
The future of airborne sulfur-containing particles in the absence of fossil fuel sulfur dioxide emissions
Abstract
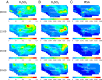
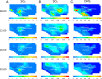
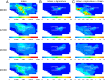
Sulfuric acid (H2SO4), formed from oxidation of sulfur dioxide (SO2) emitted during fossil fuel combustion, is a major precursor of new airborne particles, which have well-documented detrimental effects on health, air quality, and climate. Another precursor is methanesulfonic acid (MSA), produced simultaneously with SO2 during the atmospheric oxidation of organosulfur compounds (OSCs), such as dimethyl sulfide. In the present work, a multidisciplinary approach is used to examine how contributions of H2SO4 and MSA to particle formation will change in a large coastal urban area as anthropogenic fossil fuel emissions of SO2 decline. The 3-dimensional University of California Irvine-California Institute of Technology airshed model is used to compare atmospheric concentrations of gas phase MSA, H2SO4, and SO2 under current emissions of fossil fuel-associated SO2 and a best-case futuristic scenario with zero fossil fuel sulfur emissions. Model additions include results from (i) quantum chemical calculations that clarify the previously uncertain gas phase mechanism of formation of MSA and (ii) a combination of published and experimental estimates of OSC emissions, such as those from marine, agricultural, and urban processes, which include pet waste and human breath. Results show that in the zero anthropogenic SO2 emissions case, particle formation potential from H2SO4 will drop by about two orders of magnitude compared with the current situation. However, particles will continue to be generated from the oxidation of natural and anthropogenic sources of OSCs, with contributions from MSA and H2SO4 of a similar order of magnitude. This could be particularly important in agricultural areas where there are significant sources of OSCs.
Keywords: atmosphere; fossil fuel; methanesulfonic acid; new particle formation; sulfuric acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Finlayson-Pitts BJ, Pitts JN., Jr . Chemistry of the Upper and Lower Atmosphere—Theory, Experiments, and Applications. Academic Press; San Diego, CA: 2000. p. 969.
-
- IPCC . Summary for Policymakers in Climate Change. Press CU; Cambridge, UK: 2013.
-
- Pope CA, 3rd, Dockery DW. Health effects of fine particulate air pollution: Lines that connect. J Air Waste Manag Assoc. 2006;56(6):709–742. - PubMed
-
- Zhang R, Khalizov A, Wang L, Hu M, Xu W. Nucleation and growth of nanoparticles in the atmosphere. Chem Rev. 2012;112(3):1957–2011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

