Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress
- PMID: 26483456
- PMCID: PMC4640733
- DOI: 10.1073/pnas.1508347112
Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress
Abstract
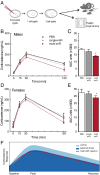
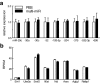
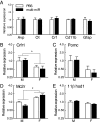
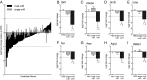
Epigenetic signatures in germ cells, capable of both responding to the parental environment and shaping offspring neurodevelopment, are uniquely positioned to mediate transgenerational outcomes. However, molecular mechanisms by which these marks may communicate experience-dependent information across generations are currently unknown. In our model of chronic paternal stress, we previously identified nine microRNAs (miRs) that were increased in the sperm of stressed sires and associated with reduced hypothalamic-pituitary-adrenal (HPA) stress axis reactivity in offspring. In the current study, we rigorously examine the hypothesis that these sperm miRs function postfertilization to alter offspring stress responsivity and, using zygote microinjection of the nine specific miRs, demonstrated a remarkable recapitulation of the offspring stress dysregulation phenotype. Further, we associated long-term reprogramming of the hypothalamic transcriptome with HPA axis dysfunction, noting a marked decreased in the expression of extracellular matrix and collagen gene sets that may reflect an underlying change in blood-brain barrier permeability. We conclude by investigating the developmental impact of sperm miRs in early zygotes with single-cell amplification technology, identifying the targeted degradation of stored maternal mRNA transcripts including sirtuin 1 and ubiquitin protein ligase E3a, two genes with established function in chromatin remodeling, and this potent regulatory function of miRs postfertilization likely initiates a cascade of molecular events that eventually alters stress reactivity. Overall, these findings demonstrate a clear mechanistic role for sperm miRs in the transgenerational transmission of paternal lifetime experiences.
Keywords: epigenetic; microRNA; paternal; stress; transgenerational.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation.J Neurosci. 2013 May 22;33(21):9003-12. doi: 10.1523/JNEUROSCI.0914-13.2013. J Neurosci. 2013. PMID: 23699511 Free PMC article. Clinical Trial.
-
Elevated paternal glucocorticoid exposure alters the small noncoding RNA profile in sperm and modifies anxiety and depressive phenotypes in the offspring.Transl Psychiatry. 2016 Jun 14;6(6):e837. doi: 10.1038/tp.2016.109. Transl Psychiatry. 2016. PMID: 27300263 Free PMC article.
-
Paternal obesity initiates metabolic disturbances in two generations of mice with incomplete penetrance to the F2 generation and alters the transcriptional profile of testis and sperm microRNA content.FASEB J. 2013 Oct;27(10):4226-43. doi: 10.1096/fj.12-224048. Epub 2013 Jul 11. FASEB J. 2013. PMID: 23845863
-
[Transgenerational transmission mediated by small non-coding RNA in sperm].Zhonghua Nan Ke Xue. 2016 Nov;22(11):1021-1024. Zhonghua Nan Ke Xue. 2016. PMID: 29281212 Review. Chinese.
-
Germ Cell Origins of Posttraumatic Stress Disorder Risk: The Transgenerational Impact of Parental Stress Experience.Biol Psychiatry. 2015 Sep 1;78(5):307-14. doi: 10.1016/j.biopsych.2015.03.018. Epub 2015 Mar 23. Biol Psychiatry. 2015. PMID: 25895429 Free PMC article. Review.
Cited by
-
Paternal overweight is associated with increased breast cancer risk in daughters in a mouse model.Sci Rep. 2016 Jun 24;6:28602. doi: 10.1038/srep28602. Sci Rep. 2016. PMID: 27339599 Free PMC article.
-
Small RNAs Gained during Epididymal Transit of Sperm Are Essential for Embryonic Development in Mice.Dev Cell. 2018 Aug 20;46(4):470-480.e3. doi: 10.1016/j.devcel.2018.06.024. Epub 2018 Jul 26. Dev Cell. 2018. PMID: 30057276 Free PMC article.
-
Parental breeding age effects on descendants' longevity interact over 2 generations in matrilines and patrilines.PLoS Biol. 2019 Nov 25;17(11):e3000556. doi: 10.1371/journal.pbio.3000556. eCollection 2019 Nov. PLoS Biol. 2019. PMID: 31765371 Free PMC article.
-
Neuroepigenetic mechanisms underlying fear extinction: emerging concepts.Psychopharmacology (Berl). 2019 Jan;236(1):133-142. doi: 10.1007/s00213-018-5084-4. Epub 2018 Nov 30. Psychopharmacology (Berl). 2019. PMID: 30506235 Free PMC article. Review.
-
Early life lessons: The lasting effects of germline epigenetic information on organismal development.Mol Metab. 2020 Aug;38:100924. doi: 10.1016/j.molmet.2019.12.004. Epub 2019 Dec 27. Mol Metab. 2020. PMID: 31974037 Free PMC article. Review.
References
-
- Van den Bergh BRH, Van Calster B, Smits T, Van Huffel S, Lagae L. Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: A prospective study on the fetal origins of depressed mood. Neuropsychopharmacology. 2008;33(3):536–545. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MH091258/MH/NIMH NIH HHS/United States
- P30-DK19525/DK/NIDDK NIH HHS/United States
- MH108286/MH/NIMH NIH HHS/United States
- R01 MH087597/MH/NIMH NIH HHS/United States
- R01 MH073030/MH/NIMH NIH HHS/United States
- R21 MH104184/MH/NIMH NIH HHS/United States
- P50 MH099910/MH/NIMH NIH HHS/United States
- R37 MH108286/MH/NIMH NIH HHS/United States
- MH073030/MH/NIMH NIH HHS/United States
- MH104184/MH/NIMH NIH HHS/United States
- MH099910/MH/NIMH NIH HHS/United States
- R01 MH091258/MH/NIMH NIH HHS/United States
- MH087597/MH/NIMH NIH HHS/United States
- P30 DK019525/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

