Erk signaling is indispensable for genomic stability and self-renewal of mouse embryonic stem cells
- PMID: 26483458
- PMCID: PMC4640739
- DOI: 10.1073/pnas.1516319112
Erk signaling is indispensable for genomic stability and self-renewal of mouse embryonic stem cells
Abstract
Inhibition of Mek/Erk signaling by pharmacological Mek inhibitors promotes self-renewal and pluripotency of mouse embryonic stem cells (ESCs). Intriguingly, Erk signaling is essential for human ESC self-renewal. Here we demonstrate that Erk signaling is critical for mouse ESC self-renewal and genomic stability. Erk-depleted ESCs cannot be maintained. Lack of Erk leads to rapid telomere shortening and genomic instability, in association with misregulated expression of pluripotency genes, reduced cell proliferation, G1 cell-cycle arrest, and increased apoptosis. Erk signaling is also required for the activation of differentiation genes but not for the repression of pluripotency genes during ESC differentiation. Furthermore, we find an Erk-independent function of Mek, which may explain the diverse effects of Mek inhibition and Erk knockout on ESC self-renewal. Together, in contrast to the prevailing view, Erk signaling is required for telomere maintenance, genomic stability, and self-renewal of mouse ESCs.
Keywords: Erk; Mek; embryonic stem cells; genomic stability; self-renewal.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


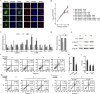




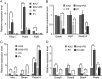

Similar articles
-
MEK/ERK signaling contributes to the maintenance of human embryonic stem cell self-renewal.Differentiation. 2007 Apr;75(4):299-307. doi: 10.1111/j.1432-0436.2006.00143.x. Epub 2007 Feb 5. Differentiation. 2007. PMID: 17286604
-
A dual role of Erk signaling in embryonic stem cells.Exp Hematol. 2016 Mar;44(3):151-6. doi: 10.1016/j.exphem.2015.12.008. Epub 2016 Jan 2. Exp Hematol. 2016. PMID: 26751246 Review.
-
ERK phosphorylates ESRRB to regulate the self-renewal and differentiation of mouse embryonic stem cells.Stem Cell Reports. 2025 Mar 11;20(3):102397. doi: 10.1016/j.stemcr.2025.102397. Epub 2025 Feb 6. Stem Cell Reports. 2025. PMID: 39919750 Free PMC article.
-
Cops5 safeguards genomic stability of embryonic stem cells through regulating cellular metabolism and DNA repair.Proc Natl Acad Sci U S A. 2020 Feb 4;117(5):2519-2525. doi: 10.1073/pnas.1915079117. Epub 2020 Jan 21. Proc Natl Acad Sci U S A. 2020. PMID: 31964807 Free PMC article.
-
Molecular basis of embryonic stem cell self-renewal: from signaling pathways to pluripotency network.Cell Mol Life Sci. 2015 May;72(9):1741-57. doi: 10.1007/s00018-015-1833-2. Epub 2015 Jan 17. Cell Mol Life Sci. 2015. PMID: 25595304 Free PMC article. Review.
Cited by
-
N6-methyladenosine (m6A) depletion regulates pluripotency exit by activating signaling pathways in embryonic stem cells.Proc Natl Acad Sci U S A. 2021 Dec 21;118(51):e2105192118. doi: 10.1073/pnas.2105192118. Proc Natl Acad Sci U S A. 2021. PMID: 34921114 Free PMC article.
-
Ground rules of the pluripotency gene regulatory network.Nat Rev Genet. 2017 Mar;18(3):180-191. doi: 10.1038/nrg.2016.156. Epub 2017 Jan 3. Nat Rev Genet. 2017. PMID: 28045100 Review.
-
Adult Neural Stem Cells: Basic Research and Production Strategies for Neurorestorative Therapy.Stem Cells Int. 2018 Apr 1;2018:4835491. doi: 10.1155/2018/4835491. eCollection 2018. Stem Cells Int. 2018. PMID: 29760724 Free PMC article. Review.
-
Noncoding telomeric repeat-containing RNA inhibits the progression of hepatocellular carcinoma by regulating telomerase-mediated telomere length.Cancer Sci. 2020 Aug;111(8):2789-2802. doi: 10.1111/cas.14442. Epub 2020 Jul 1. Cancer Sci. 2020. PMID: 32357278 Free PMC article.
-
Certain ortho-hydroxylated brominated ethers are promiscuous kinase inhibitors that impair neuronal signaling and neurodevelopmental processes.J Biol Chem. 2020 May 1;295(18):6120-6137. doi: 10.1074/jbc.RA119.011138. Epub 2020 Mar 30. J Biol Chem. 2020. PMID: 32229587 Free PMC article.
References
-
- Loh YH, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38(4):431–440. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

