Subdiffusive motion of bacteriophage in mucosal surfaces increases the frequency of bacterial encounters
- PMID: 26483471
- PMCID: PMC4640763
- DOI: 10.1073/pnas.1508355112
Subdiffusive motion of bacteriophage in mucosal surfaces increases the frequency of bacterial encounters
Abstract
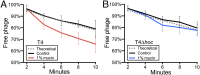
Bacteriophages (phages) defend mucosal surfaces against bacterial infections. However, their complex interactions with their bacterial hosts and with the mucus-covered epithelium remain mostly unexplored. Our previous work demonstrated that T4 phage with Hoc proteins exposed on their capsid adhered to mucin glycoproteins and protected mucus-producing tissue culture cells in vitro. On this basis, we proposed our bacteriophage adherence to mucus (BAM) model of immunity. Here, to test this model, we developed a microfluidic device (chip) that emulates a mucosal surface experiencing constant fluid flow and mucin secretion dynamics. Using mucus-producing human cells and Escherichia coli in the chip, we observed similar accumulation and persistence of mucus-adherent T4 phage and nonadherent T4∆hoc phage in the mucus. Nevertheless, T4 phage reduced bacterial colonization of the epithelium >4,000-fold compared with T4∆hoc phage. This suggests that phage adherence to mucus increases encounters with bacterial hosts by some other mechanism. Phages are traditionally thought to be completely dependent on normal diffusion, driven by random Brownian motion, for host contact. We demonstrated that T4 phage particles displayed subdiffusive motion in mucus, whereas T4∆hoc particles displayed normal diffusion. Experiments and modeling indicate that subdiffusive motion increases phage-host encounters when bacterial concentration is low. By concentrating phages in an optimal mucus zone, subdiffusion increases their host encounters and antimicrobial action. Our revised BAM model proposes that the fundamental mechanism of mucosal immunity is subdiffusion resulting from adherence to mucus. These findings suggest intriguing possibilities for engineering phages to manipulate and personalize the mucosal microbiome.
Keywords: BAM; mucus; search strategy; subdiffusion; virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Bacteriophage adhering to mucus provide a non-host-derived immunity.Proc Natl Acad Sci U S A. 2013 Jun 25;110(26):10771-6. doi: 10.1073/pnas.1305923110. Epub 2013 May 20. Proc Natl Acad Sci U S A. 2013. PMID: 23690590 Free PMC article.
-
Relevance of the bacteriophage adherence to mucus model for Pseudomonas aeruginosa phages.Microbiol Spectr. 2024 Aug 6;12(8):e0352023. doi: 10.1128/spectrum.03520-23. Epub 2024 Jun 24. Microbiol Spectr. 2024. PMID: 38912817 Free PMC article.
-
Bacteriophage Adherence to Mucus Mediates Preventive Protection against Pathogenic Bacteria.mBio. 2019 Nov 19;10(6):e01984-19. doi: 10.1128/mBio.01984-19. mBio. 2019. PMID: 31744913 Free PMC article.
-
Pulmonary bacteriophage and cystic fibrosis airway mucus: friends or foes?Front Med (Lausanne). 2023 May 17;10:1088494. doi: 10.3389/fmed.2023.1088494. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37265479 Free PMC article. Review.
-
[Does taxis exist in bacterial viruses?].Biofizika. 1995 Jan-Feb;40(1):60-73. Biofizika. 1995. PMID: 7703277 Review. Russian.
Cited by
-
Keeping It Together: Structures, Functions, and Applications of Viral Decoration Proteins.Viruses. 2020 Oct 14;12(10):1163. doi: 10.3390/v12101163. Viruses. 2020. PMID: 33066635 Free PMC article. Review.
-
Salmonella enterica Serovar Enteritidis Control in Poultry Litter Mediated by Lytic Bacteriophage Isolated from Swine Manure.Int J Environ Res Public Health. 2021 Aug 23;18(16):8862. doi: 10.3390/ijerph18168862. Int J Environ Res Public Health. 2021. PMID: 34444610 Free PMC article.
-
Bacteriophage-Bacteria Interactions in the Gut: From Invertebrates to Mammals.Annu Rev Virol. 2021 Sep 29;8(1):95-113. doi: 10.1146/annurev-virology-091919-101238. Epub 2021 Jul 13. Annu Rev Virol. 2021. PMID: 34255542 Free PMC article. Review.
-
Targeting of Mammalian Glycans Enhances Phage Predation in the Gastrointestinal Tract.mBio. 2021 Feb 9;12(1):e03474-20. doi: 10.1128/mBio.03474-20. mBio. 2021. PMID: 33563833 Free PMC article.
-
Graphene oxide nanohybrids for electron transfer-mediated antimicrobial activity.Nanoscale Adv. 2019 Aug 15;1(9):3727-3740. doi: 10.1039/c9na00272c. eCollection 2019 Sep 11. Nanoscale Adv. 2019. PMID: 36133551 Free PMC article.
References
-
- d’Hérelle F. The Bacteriophage: Its Role in Immunity. Masson et Cie; Paris: 1921.
-
- Brüssow H. Phage therapy: The Escherichia coli experience. Microbiology. 2005;151(Pt 7):2133–2140. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

