The Trk Potassium Transporter Is Required for RsmB-Mediated Activation of Virulence in the Phytopathogen Pectobacterium wasabiae
- PMID: 26483524
- PMCID: PMC4751801
- DOI: 10.1128/JB.00569-15
The Trk Potassium Transporter Is Required for RsmB-Mediated Activation of Virulence in the Phytopathogen Pectobacterium wasabiae
Abstract
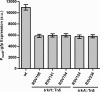
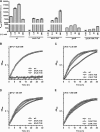
Pectobacterium wasabiae (previously known as Erwinia carotovora) is an important plant pathogen that regulates the production of plant cell wall-degrading enzymes through an N-acyl homoserine lactone-based quorum sensing system and through the GacS/GacA two-component system (also known as ExpS/ExpA). At high cell density, activation of GacS/GacA induces the expression of RsmB, a noncoding RNA that is essential for the activation of virulence in this bacterium. A genetic screen to identify regulators of RsmB revealed that mutants defective in components of a putative Trk potassium transporter (trkH and trkA) had decreased rsmB expression. Further analysis of these mutants showed that changes in potassium concentration influenced rsmB expression and consequent tissue damage in potato tubers and that this regulation required an intact Trk system. Regulation of rsmB expression by potassium via the Trk system occurred even in the absence of the GacS/GacA system, demonstrating that these systems act independently and are both required for full activation of RsmB and for the downstream induction of virulence in potato infection assays. Overall, our results identified potassium as an essential environmental factor regulating the Rsm system, and the consequent induction of virulence, in the plant pathogen P. wasabiae.
Importance: Crop losses from bacterial diseases caused by pectolytic bacteria are a major problem in agriculture. By studying the regulatory pathways involved in controlling the expression of plant cell wall-degrading enzymes in Pectobacterium wasabiae, we showed that the Trk potassium transport system plays an important role in the regulation of these pathways. The data presented further identify potassium as an important environmental factor in the regulation of virulence in this plant pathogen. We showed that a reduction in virulence can be achieved by increasing the extracellular concentration of potassium. Therefore, this work highlights how elucidation of the mechanisms involved in regulating virulence can lead to the identification of environmental factors that can influence the outcome of infection.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures




References
-
- Nykyri J, Niemi O, Koskinen P, Nokso-Koivisto J, Pasanen M, Broberg M, Plyusnin I, Törönen P, Holm L, Pirhonen M, Palva ET. 2012. Revised phylogeny and novel horizontally acquired virulence determinants of the model soft rot phytopathogen Pectobacterium wasabiae SCC3193. PLoS Pathog 8:e1003013. doi:10.1371/journal.ppat.1003013. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

