Structural and Functional Studies of a Newly Grouped Haloquadratum walsbyi Bacteriorhodopsin Reveal the Acid-resistant Light-driven Proton Pumping Activity
- PMID: 26483542
- PMCID: PMC4705956
- DOI: 10.1074/jbc.M115.685065
Structural and Functional Studies of a Newly Grouped Haloquadratum walsbyi Bacteriorhodopsin Reveal the Acid-resistant Light-driven Proton Pumping Activity
Abstract
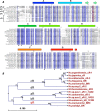
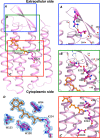
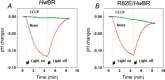
Retinal bound light-driven proton pumps are widespread in eukaryotic and prokaryotic organisms. Among these pumps, bacteriorhodopsin (BR) proteins cooperate with ATP synthase to convert captured solar energy into a biologically consumable form, ATP. In an acidic environment or when pumped-out protons accumulate in the extracellular region, the maximum absorbance of BR proteins shifts markedly to the longer wavelengths. These conditions affect the light-driven proton pumping functional exertion as well. In this study, wild-type crystal structure of a BR with optical stability under wide pH range from a square halophilic archaeon, Haloquadratum walsbyi (HwBR), was solved in two crystal forms. One crystal form, refined to 1.85 Å resolution, contains a trimer in the asymmetric unit, whereas another contains an antiparallel dimer was refined at 2.58 Å. HwBR could not be classified into any existing subgroup of archaeal BR proteins based on the protein sequence phylogenetic tree, and it showed unique absorption spectral stability when exposed to low pH values. All structures showed a unique hydrogen-bonding network between Arg(82) and Thr(201), linking the BC and FG loops to shield the retinal-binding pocket in the interior from the extracellular environment. This result was supported by R82E mutation that attenuated the optical stability. The negatively charged cytoplasmic side and the Arg(82)-Thr(201) hydrogen bond may play an important role in the proton translocation trend in HwBR under acidic conditions. Our findings have unveiled a strategy adopted by BR proteins to solidify their defenses against unfavorable environments and maintain their optical properties associated with proton pumping.
Keywords: bacteriorhodopsin; crystal structure; lipid cubic phase; membrane protein; proton pump; rhodopsin; site-directed mutagenesis; spectral-tuning; structure.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures







References
-
- Spudich J. L., Yang C. S., Jung K. H., and Spudich E. N. (2000) Retinylidene proteins: structures and functions from archaea to humans. Annu. Rev. Cell Dev. Biol. 16, 365–392 - PubMed
-
- Béjà O., Spudich E. N., Spudich J. L., Leclerc M., and DeLong E. F. (2001) Proteorhodopsin phototrophy in the ocean. Nature 411, 786–789 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

