Structural Insights into the MMACHC-MMADHC Protein Complex Involved in Vitamin B12 Trafficking
- PMID: 26483544
- PMCID: PMC4705923
- DOI: 10.1074/jbc.M115.683268
Structural Insights into the MMACHC-MMADHC Protein Complex Involved in Vitamin B12 Trafficking
Abstract
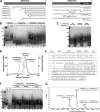
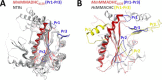
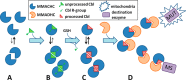
Conversion of vitamin B12 (cobalamin, Cbl) into the cofactor forms methyl-Cbl (MeCbl) and adenosyl-Cbl (AdoCbl) is required for the function of two crucial enzymes, mitochondrial methylmalonyl-CoA mutase and cytosolic methionine synthase, respectively. The intracellular proteins MMACHC and MMADHC play important roles in processing and targeting the Cbl cofactor to its destination enzymes, and recent evidence suggests that they may interact while performing these essential trafficking functions. To better understand the molecular basis of this interaction, we have mapped the crucial protein regions required, indicate that Cbl is likely processed by MMACHC prior to interaction with MMADHC, and identify patient mutations on both proteins that interfere with complex formation, via different mechanisms. We further report the crystal structure of the MMADHC C-terminal region at 2.2 Å resolution, revealing a modified nitroreductase fold with surprising homology to MMACHC despite their poor sequence conservation. Because MMADHC demonstrates no known enzymatic activity, we propose it as the first protein known to repurpose the nitroreductase fold solely for protein-protein interaction. Using small angle x-ray scattering, we reveal the MMACHC-MMADHC complex as a 1:1 heterodimer and provide a structural model of this interaction, where the interaction region overlaps with the MMACHC-Cbl binding site. Together, our findings provide novel structural evidence and mechanistic insight into an essential biological process, whereby an intracellular "trafficking chaperone" highly specific for a trace element cofactor functions via protein-protein interaction, which is disrupted by inherited disease mutations.
Keywords: crystal structure; metabolic disease; nitroreductase fold; protein-protein interaction; site-directed mutagenesis; small-angle x-ray scattering (SAXS); vitamin B12.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Watkins D., and Rosenblatt D. S. (2014) Inherited disorders of folate and cobalamin transport and metabolism. in The Online Metabolic and Molecular Bases of Inherited Disease (Valle D., Beaudet A. L., Vogelstein B., Kinzler K. W., Antonarakis S. E., Ballabio A., Gibson M., and Mitchell G., eds), McGraw-Hill, New York
-
- Baumgartner M. R. (2013) Vitamin-responsive disorders: cobalamin, folate, biotin, vitamins B1 and E. Handb. Clin. Neurol. 113, 1799–1810 - PubMed
-
- Harding C. O., Arnold G., Barness L. A., Wolff J. A., and Rosenblatt D. S. (1997) Functional methionine synthase deficiency due to cblG disorder: a report of two patients and a review. Am. J. Med. Genet. 71, 384–390 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

