NF2/Merlin mediates contact-dependent inhibition of EGFR mobility and internalization via cortical actomyosin
- PMID: 26483553
- PMCID: PMC4621825
- DOI: 10.1083/jcb.201503081
NF2/Merlin mediates contact-dependent inhibition of EGFR mobility and internalization via cortical actomyosin
Abstract
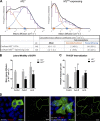
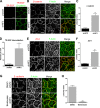
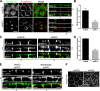
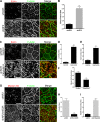
The proliferation of normal cells is inhibited at confluence, but the molecular basis of this phenomenon, known as contact-dependent inhibition of proliferation, is unclear. We previously identified the neurofibromatosis type 2 (NF2) tumor suppressor Merlin as a critical mediator of contact-dependent inhibition of proliferation and specifically found that Merlin inhibits the internalization of, and signaling from, the epidermal growth factor receptor (EGFR) in response to cell contact. Merlin is closely related to the membrane-cytoskeleton linking proteins Ezrin, Radixin, and Moesin, and localization of Merlin to the cortical cytoskeleton is required for contact-dependent regulation of EGFR. We show that Merlin and Ezrin are essential components of a mechanism whereby mechanical forces associated with the establishment of cell-cell junctions are transduced across the cell cortex via the cortical actomyosin cytoskeleton to control the lateral mobility and activity of EGFR, providing novel insight into how cells inhibit mitogenic signaling in response to cell contact.
© 2015 Chiassson-MacKenzie et al.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

