Meninges harbor cells expressing neural precursor markers during development and adulthood
- PMID: 26483637
- PMCID: PMC4591429
- DOI: 10.3389/fncel.2015.00383
Meninges harbor cells expressing neural precursor markers during development and adulthood
Abstract
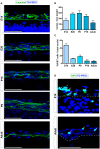
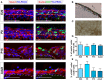
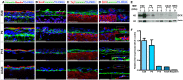
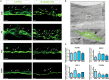
Brain and skull developments are tightly synchronized, allowing the cranial bones to dynamically adapt to the brain shape. At the brain-skull interface, meninges produce the trophic signals necessary for normal corticogenesis and bone development. Meninges harbor different cell populations, including cells forming the endosteum of the cranial vault. Recently, we and other groups have described the presence in meninges of a cell population endowed with neural differentiation potential in vitro and, after transplantation, in vivo. However, whether meninges may be a niche for neural progenitor cells during embryonic development and in adulthood remains to be determined. In this work we provide the first description of the distribution of neural precursor markers in rat meninges during development up to adulthood. We conclude that meninges share common properties with the classical neural stem cell niche, as they: (i) are a highly proliferating tissue; (ii) host cells expressing neural precursor markers such as nestin, vimentin, Sox2 and doublecortin; and (iii) are enriched in extracellular matrix components (e.g., fractones) known to bind and concentrate growth factors. This study underlines the importance of meninges as a potential niche for endogenous precursor cells during development and in adulthood.
Keywords: brain development; fractones; meninges; nestin; neural precursor cells; neural stem cell niche; proliferation.
Figures

References
-
- Belmadani A., Ren D., Bhattacharyya B. J., Hope T. J., Perlman H., Miller R. J. (2015). Identification of a sustained neurogenic zone at the dorsal surface of the adult mouse hippocampus and its regulation by the chemokine SDF-1. Hippocampus. 10.1002/hipo.22428. [Epub ahead of print]. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

