Corticofugal projection patterns of whisker sensorimotor cortex to the sensory trigeminal nuclei
- PMID: 26483640
- PMCID: PMC4588702
- DOI: 10.3389/fncir.2015.00053
Corticofugal projection patterns of whisker sensorimotor cortex to the sensory trigeminal nuclei
Abstract
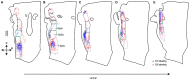
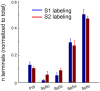
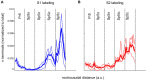
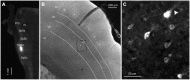
The primary (S1) and secondary (S2) somatosensory cortices project to several trigeminal sensory nuclei. One putative function of these corticofugal projections is the gating of sensory transmission through the trigeminal principal nucleus (Pr5), and some have proposed that S1 and S2 project differentially to the spinal trigeminal subnuclei, which have inhibitory circuits that could inhibit or disinhibit the output projections of Pr5. Very little, however, is known about the origin of sensorimotor corticofugal projections and their patterns of termination in the various trigeminal nuclei. We addressed this issue by injecting anterograde tracers in S1, S2 and primary motor (M1) cortices, and quantitatively characterizing the distribution of labeled terminals within the entire rostro-caudal chain of trigeminal sub-nuclei. We confirmed our anterograde tracing results by injecting retrograde tracers at various rostro-caudal levels within the trigeminal sensory nuclei to determine the position of retrogradely labeled cortical cells with respect to S1 barrel cortex. Our results demonstrate that S1 and S2 projections terminate in largely overlapping regions but show some significant differences. Whereas S1 projection terminals tend to cluster within the principal trigeminal (Pr5), caudal spinal trigeminal interpolaris (Sp5ic), and the dorsal spinal trigeminal caudalis (Sp5c), S2 projection terminals are distributed in a continuum across all trigeminal nuclei. Contrary to the view that sensory gating could be mediated by differential activation of inhibitory interconnections between the spinal trigeminal subnuclei, we observed that projections from S1 and S2 are largely overlapping in these subnuclei despite the differences noted earlier.
Keywords: anterograde tracing; barrel cortex; corticofugal pathways; retrograde tracing; trigeminal nuclei; whisker.
Figures





Similar articles
-
Collateral projection from the locus coeruleus to whisker-related sensory and motor brain regions of the rat.J Comp Neurol. 2009 Jun 1;514(4):387-402. doi: 10.1002/cne.22012. J Comp Neurol. 2009. PMID: 19330821
-
Common fur and mystacial vibrissae parallel sensory pathways: 14 C 2-deoxyglucose and WGA-HRP studies in the rat.J Comp Neurol. 1988 Apr 15;270(3):446-69. doi: 10.1002/cne.902700312. J Comp Neurol. 1988. PMID: 3372744
-
Dual projections of tuberomammillary neurons to whisker-related, sensory and motor regions of the rat.Brain Res. 2010 Oct 1;1354:64-73. doi: 10.1016/j.brainres.2010.07.093. Epub 2010 Aug 2. Brain Res. 2010. PMID: 20682294
-
Long-range connectivity of mouse primary somatosensory barrel cortex.Eur J Neurosci. 2010 Jun;31(12):2221-33. doi: 10.1111/j.1460-9568.2010.07264.x. Epub 2010 Jun 9. Eur J Neurosci. 2010. PMID: 20550566 Review.
-
Cortical control of whisker movement.Annu Rev Neurosci. 2014;37:183-203. doi: 10.1146/annurev-neuro-062012-170344. Epub 2014 May 9. Annu Rev Neurosci. 2014. PMID: 24821429 Review.
Cited by
-
The trigeminal pathways.J Neurol. 2022 Jul;269(7):3443-3460. doi: 10.1007/s00415-022-11002-4. Epub 2022 Mar 6. J Neurol. 2022. PMID: 35249132 Review.
-
Primary Tactile Thalamus Spiking Reflects Cognitive Signals.J Neurosci. 2018 May 23;38(21):4870-4885. doi: 10.1523/JNEUROSCI.2403-17.2018. Epub 2018 Apr 27. J Neurosci. 2018. PMID: 29703788 Free PMC article.
-
Organization and development of bilateral somatosensory feedback projections in mice.iScience. 2025 May 21;28(6):112725. doi: 10.1016/j.isci.2025.112725. eCollection 2025 Jun 20. iScience. 2025. PMID: 40538433 Free PMC article.
-
Single-axon level morphological analysis of corticofugal projection neurons in mouse barrel field.Sci Rep. 2017 Jun 6;7(1):2846. doi: 10.1038/s41598-017-03000-8. Sci Rep. 2017. PMID: 28588276 Free PMC article.
-
Orofacial Movements Involve Parallel Corticobulbar Projections from Motor Cortex to Trigeminal Premotor Nuclei.Neuron. 2019 Nov 20;104(4):765-780.e3. doi: 10.1016/j.neuron.2019.08.032. Epub 2019 Oct 3. Neuron. 2019. PMID: 31587918 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

