Systemic Sclerosis Patients Present Alterations in the Expression of Molecules Involved in B-Cell Regulation
- PMID: 26483788
- PMCID: PMC4586944
- DOI: 10.3389/fimmu.2015.00496
Systemic Sclerosis Patients Present Alterations in the Expression of Molecules Involved in B-Cell Regulation
Abstract
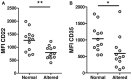
The activation threshold of B cells is tightly regulated by an array of inhibitory and activator receptors in such a way that disturbances in their expression can lead to the appearance of autoimmunity. The aim of this study was to evaluate the expression of activating and inhibitory molecules involved in the modulation of B cell functions in transitional, naive, and memory B-cell subpopulations from systemic sclerosis patients. To achieve this, blood samples were drawn from 31 systemic sclerosis patients and 53 healthy individuals. Surface expression of CD86, MHC II, CD19, CD21, CD40, CD22, Siglec 10, CD35, and FcγRIIB was determined by flow cytometry. IL-10 production was evaluated by intracellular flow cytometry from isolated B cells. Soluble IL-6 and IL-10 levels were measured by ELISA from supernatants of stimulated B cells. Systemic sclerosis patients exhibit an increased frequency of transitional and naive B cells related to memory B cells compared with healthy controls. Transitional and naive B cells from patients express higher levels of CD86 and FcγRIIB than healthy donors. Also, B cells from patients show high expression of CD19 and CD40, whereas memory cells from systemic sclerosis patients show reduced expression of CD35. CD19 and CD35 expression levels associate with different autoantibody profiles. IL-10(+) B cells and secreted levels of IL-10 were markedly reduced in patients. In conclusion, systemic sclerosis patients show alterations in the expression of molecules involved in B-cell regulation. These abnormalities may be determinant in the B-cell hyperactivation observed in systemic sclerosis.
Keywords: FcγRIIb; IL-10; Siglec; regulatory B cells; systemic sclerosis.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

