The hypocretins (orexins) mediate the "phasic" components of REM sleep: A new hypothesis
- PMID: 26483897
- PMCID: PMC4521687
- DOI: 10.1016/j.slsci.2014.07.021
The hypocretins (orexins) mediate the "phasic" components of REM sleep: A new hypothesis
Abstract
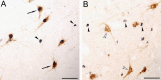
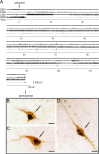
In 1998, a group of phenotypically distinct neurons were discovered in the postero-lateral hypothalamus which contained the neuropeptides hypocretin 1 and hypocretin 2 (also called orexin A and orexin B), which are excitatory neuromodulators. Hypocretinergic neurons project throughout the central nervous system and have been involved in the generation and maintenance of wakefulness. The sleep disorder narcolepsy, characterized by hypersomnia and cataplexy, is produced by degeneration of these neurons. The hypocretinergic neurons are active during wakefulness in conjunction with the presence of motor activity that occurs during survival-related behaviors. These neurons decrease their firing rate during non-REM sleep; however there is still controversy upon the activity and role of these neurons during REM sleep. Hence, in the present report we conducted a critical review of the literature of the hypocretinergic system during REM sleep, and hypothesize a possible role of this system in the generation of REM sleep.
Keywords: Cataplexy; Hypothalamus; MCH; Narcolepsy; Paradoxical sleep; Peptides.
Figures




Similar articles
-
Hypocretinergic and non-hypocretinergic projections from the hypothalamus to the REM sleep executive area of the pons.Brain Res. 2013 Jan 23;1491:68-77. doi: 10.1016/j.brainres.2012.10.050. Epub 2012 Oct 30. Brain Res. 2013. PMID: 23122879 Free PMC article.
-
MCH-containing neurons in the hypothalamus of the cat: searching for a role in the control of sleep and wakefulness.Brain Res. 2006 Nov 13;1119(1):101-14. doi: 10.1016/j.brainres.2006.08.100. Epub 2006 Oct 9. Brain Res. 2006. PMID: 17027934 Free PMC article.
-
Rapid eye movement sleep behavior disorder and rapid eye movement sleep without atonia in narcolepsy.Sleep Med. 2013 Aug;14(8):775-81. doi: 10.1016/j.sleep.2012.10.006. Epub 2012 Dec 5. Sleep Med. 2013. PMID: 23219054
-
[Modulation by the hypocretinergic/orexinergic neurotransmission system in sleep-wakefulness cycle states].Rev Neurol. 2007 Oct 16-31;45(8):482-90. Rev Neurol. 2007. PMID: 17948215 Review. Spanish.
-
[Hypocretins: involvement in the regulation of sleep-wakefulness cycle and pathogenesis of narcolepsy].Postepy Hig Med Dosw (Online). 2007;61:1-12. Postepy Hig Med Dosw (Online). 2007. PMID: 17245312 Review. Polish.
Cited by
-
Subchronical treatment with Fluoxetine modifies the activity of the MCHergic and hypocretinergic systems. Evidences from peptide CSF concentration and gene expression.Sleep Sci. 2016 Apr-Jun;9(2):89-93. doi: 10.1016/j.slsci.2016.05.010. Epub 2016 Jun 7. Sleep Sci. 2016. PMID: 27656272 Free PMC article.
-
Neural Control of the Upper Airway: Respiratory and State-Dependent Mechanisms.Compr Physiol. 2016 Sep 15;6(4):1801-1850. doi: 10.1002/cphy.c160002. Compr Physiol. 2016. PMID: 27783860 Free PMC article. Review.
-
Hypoxia and hypercapnia inhibit hypothalamic orexin neurons in rats.J Neurophysiol. 2016 Nov 1;116(5):2250-2259. doi: 10.1152/jn.00196.2016. Epub 2016 Aug 24. J Neurophysiol. 2016. PMID: 27559138 Free PMC article.
-
Melanin-Concentrating Hormone (MCH): Role in REM Sleep and Depression.Front Neurosci. 2015 Dec 17;9:475. doi: 10.3389/fnins.2015.00475. eCollection 2015. Front Neurosci. 2015. PMID: 26733789 Free PMC article. Review.
-
Inactivation of hypocretin receptor-2 signaling in dopaminergic neurons induces hyperarousal and enhanced cognition but impaired inhibitory control.Mol Psychiatry. 2024 Feb;29(2):327-341. doi: 10.1038/s41380-023-02329-z. Epub 2023 Dec 21. Mol Psychiatry. 2024. PMID: 38123729 Free PMC article.
References
-
- Mignot E. Narcolepsy: pathophysiology and genetic predisposition. In: Krieger M.H., Roth T., Dement W., editors. Principles and practices of sleep medicine. Saunders; Philadelphia: 2011. pp. 938–956.
-
- De la Herran-Arita A.K., Kornum B.R., Mahlios J., Jiang W., Lin L., Hou T. CD4+ T cell autoimmunity to hypocretin/orexin and cross-reactivity to a 2009 H1N1 influenza A epitope in narcolepsy. Sci Transl Med. 2013;5:216ra176. - PubMed
-
- Chase M.H. Motor control during sleep and wakefulness: clarifying controversies and resolving paradoxes. Sleep Med Rev. 2013;17:299–312. - PubMed
-
- Luppi P.H., Clement O., Fort P. Paradoxical (REM) sleep genesis by the brainstem is under hypothalamic control. Curr Opin Neurobiol. 2013;23:786–792. - PubMed
-
- Siegel J.M. REM sleep. In: Kryger M.H., Roth T., Dement W.C., editors. Principles and practices of sleep medicine. Elsevier-Saunders; Philadelphia: 2005. pp. 120–135.
LinkOut - more resources
Full Text Sources
Other Literature Sources

