PARP1 Inhibitors: antitumor drug design
- PMID: 26483957
- PMCID: PMC4610162
PARP1 Inhibitors: antitumor drug design
Abstract
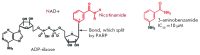
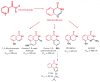
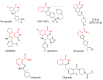
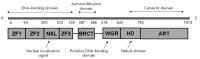
The poly (ADP-ribose) polymerase 1 (PARP1) enzyme is one of the promising molecular targets for the discovery of antitumor drugs. PARP1 is a common nuclear protein (1-2 million molecules per cell) serving as a "sensor" for DNA strand breaks. Increased PARP1 expression is sometimes observed in melanomas, breast cancer, lung cancer, and other neoplastic diseases. The PARP1 expression level is a prognostic indicator and is associated with a poor survival prognosis. There is evidence that high PARP1 expression and treatment-resistance of tumors are correlated. PARP1 inhibitors are promising antitumor agents, since they act as chemo- and radiosensitizers in the conventional therapy of malignant tumors. Furthermore, PARP1 inhibitors can be used as independent, effective drugs against tumors with broken DNA repair mechanisms. Currently, third-generation PARP1 inhibitors are being developed, many of which are undergoing Phase II clinical trials. In this review, we focus on the properties and features of the PARP1 inhibitors identified in preclinical and clinical trials. We also describe some problems associated with the application of PARP1 inhibitors. The possibility of developing new PARP1 inhibitors aimed at DNA binding and transcriptional activity rather than the catalytic domain of the protein is discussed.
Keywords: PARP1 inhibitors; antitumor agents; poly (ADP-ribose) polymerase 1.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
