Clinical Application of Multigene Panels: Challenges of Next-Generation Counseling and Cancer Risk Management
- PMID: 26484312
- PMCID: PMC4586434
- DOI: 10.3389/fonc.2015.00208
Clinical Application of Multigene Panels: Challenges of Next-Generation Counseling and Cancer Risk Management
Erratum in
-
Corrigendum: Clinical Application of Multigene Panels: Challenges of Next-Generation Counseling and Cancer Risk Management.Front Oncol. 2015 Dec 2;5:271. doi: 10.3389/fonc.2015.00271. eCollection 2015. Front Oncol. 2015. PMID: 26697406 Free PMC article.
Abstract
Background: Multigene panels can be a cost- and time-effective alternative to sequentially testing multiple genes, especially with a mixed family cancer phenotype. However, moving beyond our single-gene testing paradigm has unveiled many new challenges to the clinician. The purpose of this article is to familiarize the reader with some of the challenges, as well as potential opportunities, of expanded hereditary cancer panel testing.
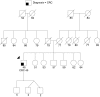
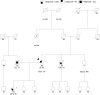
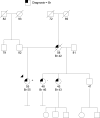
Methods: We include results from 348 commercial multigene panel tests ordered from January 1, 2014, through October 1, 2014, by clinicians associated with the City of Hope's Clinical Cancer Genetics Community of Practice. We also discuss specific challenging cases that arose during this period involving abnormalities in the genes: CDH1, TP53, PMS2, PALB2, CHEK2, NBN, and RAD51C.
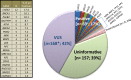
Results: If historically high risk genes only were included in the panels (BRCA1, BRCA2, MSH6, PMS2, TP53, APC, CDH1), the results would have been positive only 6.2% of the time, instead of 17%. Results returned with variants of uncertain significance (VUS) 42% of the time.
Conclusion: These figures and cases stress the importance of adequate pre-test counseling in anticipation of higher percentages of positive, VUS, unexpected, and ambiguous test results. Test result ambiguity can be limited by the use of phenotype-specific panels; if found, multiple resources (the literature, reference laboratory, colleagues, national experts, and research efforts) can be accessed to better clarify counseling and management for the patient and family. For pathogenic variants in low and moderate risk genes, empiric risk modeling based on the patient's personal and family history of cancer may supersede gene-specific risk. Commercial laboratory and patient contributions to public databases and research efforts will be needed to better classify variants and reduce clinical ambiguity of multigene panels.
Keywords: genetic counseling; hereditary breast cancer; hereditary cancer panel; hereditary colon cancer; multigene panels; next-generation sequencing.
Figures

References
-
- Halbert CH, Wenzel L, Lerman C, Peshkin BN, Narod S, Marcus A, et al. Predictors of participation in psychosocial telephone counseling following genetic testing for BRCA1 and BRCA2 mutations. Cancer Epidemiol Biomarkers Prev (2004) 13(5):875–81. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

