Antiviral Potential of Algae Polysaccharides Isolated from Marine Sources: A Review
- PMID: 26484353
- PMCID: PMC4592888
- DOI: 10.1155/2015/825203
Antiviral Potential of Algae Polysaccharides Isolated from Marine Sources: A Review
Abstract
From food to fertilizer, algal derived products are largely employed in assorted industries, including agricultural, biomedical, food, and pharmaceutical industries. Among different chemical compositions isolated from algae, polysaccharides are the most well-established compounds, which were subjected to a variety of studies due to extensive bioactivities. Over the past few decades, the promising results for antiviral potential of algae-derived polysaccharides have advocated them as inordinate candidates for pharmaceutical research. Numerous studies have isolated various algal polysaccharides possessing antiviral activities, including carrageenan, alginate, fucan, laminaran, and naviculan. In addition, different mechanisms of action have been reported for these polysaccharides, such as inhibiting the binding or internalization of virus into the host cells or suppressing DNA replication and protein synthesis. This review strives for compiling previous antiviral studies of algae-derived polysaccharides and their mechanism of action towards their development as natural antiviral agents for future investigations.
Figures
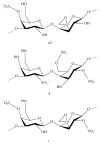
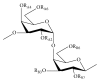

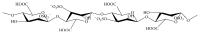

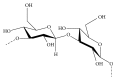
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

