Molecular effects of Lapatinib in the treatment of HER2 overexpressing oesophago-gastric adenocarcinoma
- PMID: 26484410
- PMCID: PMC4815800
- DOI: 10.1038/bjc.2015.342
Molecular effects of Lapatinib in the treatment of HER2 overexpressing oesophago-gastric adenocarcinoma
Abstract
Background: Lapatinib, a dual EGFR and HER2 inhibitor has shown disappointing results in clinical trials of metastatic oesophago-gastric adenocarcinomas (OGAs), and in vitro studies suggest that MET, IGFR, and HER3 confer resistance. This trial applied Lapatinib in the curative neoadjuvant setting and investigated the feasibility and utility of additional endoscopy and biopsy for assessment of resistance mechanisms ex vivo and in vivo.
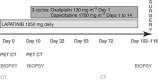
Methods: Patients with HER2 overexpressing OGA were treated for 10 days with Lapatinib monotherapy, and then in combination with three cycles of Oxaliplatin and Capecitabine before surgery. Endoscopic samples were taken for molecular analysis at: baseline including for ex vivo culture +/- Lapatinib to predict in vivo response, post-Lapatinib monotherapy and at surgery. Immunohistochemistry (IHC) and proteomic analysis was performed to assess cell kinetics and signalling activity.
Results: The trial closed early (n=10) due to an anastomotic leak in two patients for which a causative effect of Lapatinib could not be excluded. The reduction in Phosphorylated-HER2 (P-HER2) and P-EGFR in the ex vivo-treated biopsy demonstrated good correlation with the in vivo response at day 10. Proteomic analysis pre and post-Lapatinib demonstrated target inhibition (P-ERBB2, P-EGFR, P-PI3K, P-AKT, and P-ERK) that persisted until surgery. There was also significant correlation between the activation of MET with the level of P-Erk (P=0.0005) and P-PI3K : T-PI3K (total PI3K) ratio (P=0.0037). There was no significant correlation between the activation status of IGFR and HER3 with downstream signalling molecules.
Conclusions: Additional endoscopy and biopsy sampling for multiple biomarker endpoints was feasible and confirmed in vitro data that MET is likely to be a significant mechanism of Lapatinib resistance in vivo.
Figures



Similar articles
-
Functional genetic approach identifies MET, HER3, IGF1R, INSR pathways as determinants of lapatinib unresponsiveness in HER2-positive gastric cancer.Clin Cancer Res. 2014 Sep 1;20(17):4559-73. doi: 10.1158/1078-0432.CCR-13-3396. Epub 2014 Jun 27. Clin Cancer Res. 2014. PMID: 24973425
-
Lapatinib in Combination With Capecitabine Plus Oxaliplatin in Human Epidermal Growth Factor Receptor 2-Positive Advanced or Metastatic Gastric, Esophageal, or Gastroesophageal Adenocarcinoma: TRIO-013/LOGiC--A Randomized Phase III Trial.J Clin Oncol. 2016 Feb 10;34(5):443-51. doi: 10.1200/JCO.2015.62.6598. Epub 2015 Nov 30. J Clin Oncol. 2016. PMID: 26628478 Clinical Trial.
-
Lapatinib versus lapatinib plus capecitabine as second-line treatment in human epidermal growth factor receptor 2-amplified metastatic gastro-oesophageal cancer: a randomised phase II trial of the Arbeitsgemeinschaft Internistische Onkologie.Eur J Cancer. 2015 Mar;51(5):569-76. doi: 10.1016/j.ejca.2015.01.059. Epub 2015 Feb 16. Eur J Cancer. 2015. PMID: 25694417 Clinical Trial.
-
Lapatinib: a dual inhibitor of human epidermal growth factor receptor tyrosine kinases.Clin Ther. 2008 Aug;30(8):1426-47. doi: 10.1016/j.clinthera.2008.08.008. Clin Ther. 2008. PMID: 18803986 Review.
-
Update on Gastroesophageal Adenocarcinoma Targeted Therapies.Hematol Oncol Clin North Am. 2017 Jun;31(3):511-527. doi: 10.1016/j.hoc.2017.01.009. Hematol Oncol Clin North Am. 2017. PMID: 28501091 Free PMC article. Review.
Cited by
-
Active Components of Fungus Shiraia bambusiscola Can Specifically Induce BGC823 Gastric Cancer Cell Apoptosis.Cell J. 2016 Jul-Sep;18(2):149-58. doi: 10.22074/cellj.2016.4309. Epub 2016 May 30. Cell J. 2016. PMID: 27540519 Free PMC article.
-
Long noncoding RNA NONHSAT160169.1 promotes resistance via hsa-let-7c-3p/SOX2 axis in gastric cancer.Sci Rep. 2023 Nov 27;13(1):20858. doi: 10.1038/s41598-023-47961-5. Sci Rep. 2023. PMID: 38012281 Free PMC article.
-
Development and validation of a survival model for esophageal adenocarcinoma based on autophagy-associated genes.Bioengineered. 2021 Dec;12(1):3434-3454. doi: 10.1080/21655979.2021.1946235. Bioengineered. 2021. PMID: 34252349 Free PMC article.
-
Combination effect of lapatinib with foretinib in HER2 and MET co-activated experimental esophageal adenocarcinoma.Sci Rep. 2019 Nov 26;9(1):17608. doi: 10.1038/s41598-019-54129-7. Sci Rep. 2019. PMID: 31772236 Free PMC article.
-
Immune activation by DNA damage predicts response to chemotherapy and survival in oesophageal adenocarcinoma.Gut. 2019 Nov;68(11):1918-1927. doi: 10.1136/gutjnl-2018-317624. Epub 2019 Mar 9. Gut. 2019. PMID: 30852560 Free PMC article.
References
-
- Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE (2009) Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 27: 5062–5067. - PubMed
-
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Ruschoff J, Kang YK (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376: 687–697. - PubMed
-
- CancerResearchUK (2015) Oesophageal cancer incidence statistics Cancer Research UK. Available at: http://www.cancerresearchuk.org/cancer-info/cancerstats/types/oesophagus... (accessed on 18 May 2015).
-
- Chadwick G, Groene O, Cromwell D, Hardwich R, Riley S, Crosby T, Greenaway K (2013) National Oesophago-Gastric Audit 2013 In: England, T. R. C. O. S. O. (ed.) London.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

