Targeting mitochondrial RNA polymerase in acute myeloid leukemia
- PMID: 26484416
- PMCID: PMC4741925
- DOI: 10.18632/oncotarget.6129
Targeting mitochondrial RNA polymerase in acute myeloid leukemia
Abstract
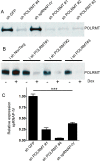
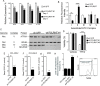
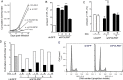
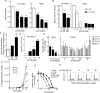
Acute myeloid leukemia (AML) cells have high oxidative phosphorylation and mitochondrial mass and low respiratory chain spare reserve capacity. We reasoned that targeting the mitochondrial RNA polymerase (POLRMT), which indirectly controls oxidative phosphorylation, represents a therapeutic strategy for AML. POLRMT-knockdown OCI-AML2 cells exhibited decreased mitochondrial gene expression, decreased levels of assembled complex I, decreased levels of mitochondrially-encoded Cox-II and decreased oxidative phosphorylation. POLRMT-knockdown cells exhibited an increase in complex II of the electron transport chain, a complex comprised entirely of subunits encoded by nuclear genes, and POLRMT-knockdown cells were resistant to a complex II inhibitor theonyltrifluoroacetone. POLRMT-knockdown cells showed a prominent increase in cell death. Treatment of OCI-AML2 cells with 10-50 µM 2-C-methyladenosine (2-CM), a chain terminator of mitochondrial transcription, reduced mitochondrial gene expression and oxidative phosphorylation, and increased cell death in a concentration-dependent manner. Treatment of normal human hematopoietic cells with 2-CM at concentrations of up to 100 µMdid not alter clonogenic growth, suggesting a therapeutic window. In an OCI-AML2 xenograft model, treatment with 2-CM (70 mg/kg, i.p., daily) decreased the volume and mass of tumours to half that of vehicle controls. 2-CM did not cause toxicity to major organs. Overall, our results in a preclinical model contribute to the functional validation of the utility of targeting the mitochondrial RNA polymerase as a therapeutic strategy for AML.
Keywords: acute myeloid leukemia; electron transport chain; mitochondria; mitochondrial RNA polymerase; oxidative phosphorylation.
Conflict of interest statement
The authors disclose no potential conflicts of interest.
Figures
Similar articles
-
The thymidine dideoxynucleoside analog, alovudine, inhibits the mitochondrial DNA polymerase γ, impairs oxidative phosphorylation and promotes monocytic differentiation in acute myeloid leukemia.Haematologica. 2019 May;104(5):963-972. doi: 10.3324/haematol.2018.195172. Epub 2018 Dec 20. Haematologica. 2019. PMID: 30573504 Free PMC article.
-
Quercetin induces mitochondrial-derived apoptosis via reactive oxygen species-mediated ERK activation in HL-60 leukemia cells and xenograft.Arch Toxicol. 2015 Jul;89(7):1103-17. doi: 10.1007/s00204-014-1300-0. Epub 2014 Aug 20. Arch Toxicol. 2015. PMID: 25138434
-
Design, Synthesis and Biological Evaluation of POLRMT Inhibitors for the Treatment of Acute Myeloid Leukemia.Chem Biol Drug Des. 2025 May;105(5):e70127. doi: 10.1111/cbdd.70127. Chem Biol Drug Des. 2025. PMID: 40413625
-
Human mitochondrial RNA polymerase: structure-function, mechanism and inhibition.Biochim Biophys Acta. 2012 Sep-Oct;1819(9-10):948-60. doi: 10.1016/j.bbagrm.2012.04.002. Epub 2012 Apr 19. Biochim Biophys Acta. 2012. PMID: 22551784 Review.
-
Targeting mitochondrial respiration for the treatment of acute myeloid leukemia.Biochem Pharmacol. 2020 Dec;182:114253. doi: 10.1016/j.bcp.2020.114253. Epub 2020 Oct 2. Biochem Pharmacol. 2020. PMID: 33011159 Free PMC article. Review.
Cited by
-
Metabolism in acute myeloid leukemia: mechanistic insights and therapeutic targets.Blood. 2023 Mar 9;141(10):1119-1135. doi: 10.1182/blood.2022018092. Blood. 2023. PMID: 36548959 Free PMC article. Review.
-
Targeting POLRMT by IMT1 inhibits colorectal cancer cell growth.Cell Death Dis. 2024 Sep 3;15(9):643. doi: 10.1038/s41419-024-07023-8. Cell Death Dis. 2024. PMID: 39227564 Free PMC article.
-
Multi-focal control of mitochondrial gene expression by oncogenic MYC provides potential therapeutic targets in cancer.Oncotarget. 2016 Nov 8;7(45):72395-72414. doi: 10.18632/oncotarget.11718. Oncotarget. 2016. PMID: 27590350 Free PMC article.
-
TRIM36 suppresses cell growth and promotes apoptosis in human esophageal squamous cell carcinoma cells by inhibiting Wnt/β-catenin signaling pathway.Hum Cell. 2022 Sep;35(5):1487-1498. doi: 10.1007/s13577-022-00737-x. Epub 2022 Jun 29. Hum Cell. 2022. PMID: 35768649
-
Glycogen homeostasis and mtDNA expression require motor neuron to muscle TGFβ/Activin Signaling in Drosophila.bioRxiv [Preprint]. 2024 Jul 31:2024.06.25.600699. doi: 10.1101/2024.06.25.600699. bioRxiv. 2024. Update in: iScience. 2024 Dec 16;28(1):111611. doi: 10.1016/j.isci.2024.111611. PMID: 39131342 Free PMC article. Updated. Preprint.
References
-
- Skrtic M, Sriskanthadevan S, Jhas B, Gebbia M, Wang X, Wang Z, Hurren R, Jitkova Y, Gronda M, Maclean N, Lai CK, Eberhard Y, Bartoszko J, Spagnuolo P, Rutledge AC, Datti A, et al. Inhibition of Mitochondrial Translation as a Therapeutic Strategy for Human Acute Myeloid Leukemia. Cancer Cell. 2011;20(5):674–688. - PMC - PubMed
-
- Caro P, Kishan AU, Norberg E, Stanley IA, Chapuy B, Ficarro SB, Polak K, Tondera D, Gounarides J, Yin H, Zhou F, Green MR, Chen L, Monti S, Marto JA, Shipp MA, et al. Metabolic signatures uncover distinct targets in molecular subsets of diffuse large B cell lymphoma. Cancer Cell. 2012;22(4):547–560. - PMC - PubMed
-
- Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ, Minhajuddin M, Ashton JM, Pei S, Grose V, O'Dwyer KM, Liesveld JL, Brookes PS, Becker MW, Jordan CT. BCL-2 Inhibition Targets Oxidative Phosphorylation and Selectively Eradicates Quiescent Human Leukemia Stem Cells. Cell Stem Cell. 2013;12(3):329–341. - PMC - PubMed
-
- Leonard JV, Schapira AH. Mitochondrial respiratory chain disorders I: mitochondrial DNA defects. Lancet. 2000;355(9200):299–304. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

