Mechanisms of adaptation and progression in idiosyncratic drug induced liver injury, clinical implications
- PMID: 26484420
- PMCID: PMC4718752
- DOI: 10.1111/liv.12988
Mechanisms of adaptation and progression in idiosyncratic drug induced liver injury, clinical implications
Abstract
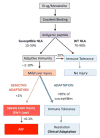
In the past decade our understanding of idiosyncratic drug induced liver injury (IDILI) and the contribution of genetic susceptibility and the adaptive immune system to the pathogenesis of this disease process has grown tremendously. One of the characteristics of IDILI is that it occurs rarely and only in a subset of individuals with a presumed susceptibility to the drug. Despite a clear association between single nucleotide polymorphisms in human leukocyte antigen (HLA) genes and certain drugs that cause IDILI, not all individuals with susceptible HLA genotypes develop clinically significant liver injury when exposed to drugs. The adaptation hypothesis has been put forth as an explanation for why only a small percentage of susceptible individuals develop overt IDILI and severe injury, while the majority with susceptible genotypes develop only mild abnormalities that resolve spontaneously upon continuation of the drug. This spontaneous resolution is referred to as clinical adaptation. Failure to adapt or defective adaptation leads to clinically significant liver injury. In this review we explore the immuno-tolerant microenvironment of the liver and the mechanisms of clinical adaptation in IDILI with a focus on the role of immune-tolerance and cellular adaptive responses.
Keywords: T cells; drug induced liver injury; hepatotoxicity; human leukocyte antigen; immune-tolerance.
© 2015 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
Conflict of Interest: No direct conflicts with this review. However, NK consults for the following pharmaceutical companies: Takeda, GSK, Pfizer, Daiichi-Sankyo, Johnson& Johnson, Geron and Ono.
Figures


References
-
- KNOLLE PA, GERKEN G. Local control of the immune response in the liver. Immunol Rev. 2000;174:21–34. - PubMed
-
- WILLIAMS GM, IATROPOULOS MJ. Alteration of liver cell function and proliferation: differentiation between adaptation and toxicity. Toxicol Pathol. 2002;30(1):41–53. - PubMed
-
- CANTOR HM, DUMONT AE. Hepatic suppression of sensitization to antigen absorbed into the portal system. Nature. 1967;215(5102):744–5. - PubMed
-
- CALLERY MP, KAMEI T, FLYE MW. The effect of portacaval shunt on delayed-hypersensitivity responses following antigen feeding. J Surg Res. 1989;46(4):391–4. - PubMed
-
- YANG R, LIU Q, GROSFELD JL, PESCOVITZ MD. Intestinal venous drainage through the liver is a prerequisite for oral tolerance induction. Journal of pediatric surgery. 1994;29(8):1145–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

