Safety and efficacy of a novel iopromide-based paclitaxel-eluting balloon following bare metal stent implantation in rabbit aorta abdominalis
- PMID: 26484558
- PMCID: PMC4923742
- DOI: 10.3233/BME-151551
Safety and efficacy of a novel iopromide-based paclitaxel-eluting balloon following bare metal stent implantation in rabbit aorta abdominalis
Abstract
Background: Drug-eluting balloons (DEB) may be promising technology for treating atherosclerotic arterial disease. In fact, several DEBs have been clinically available for the treatment of coronary in-stent restenosis (ISR), de novo coronary lesions, and peripheral artery disease.
Objective: We sought to elucidate the mechanism of action and in vivo safety and efficacy of a novel iopromide-based paclitaxel-eluting balloon.
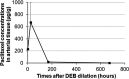
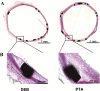
Methods: In vitro cytotoxicity of a novel DEB on human umbilical vein endothelial cells (HUVECs) and in vivo pharmacokinetics of DEB in a rabbit aorta abdominalis were assessed. Then, bare metal stents (BMS) were implanted at both the proximal and distal sites of the rabbit aorta abdominalis. Stented vascular segments were immediately dilated with a bare balloon (control group) or the DEB (DEB group) randomly. Histological evaluation was performed in all treated segments at 28 days. Because paclitaxel is a tubulin-disrupting agent that binds preferentially to β-tubulin, we measured β-tubulin expression in aortal stent specimens via immunohistochemistry.
Results: We observed that DEB was compatible and could reduce neointimal hyperplasia compared with the bare balloon. Meanwhile, immunohistochemistry revealed that β-tubulin expression in the DEB group increased compared with the control group, indirectly suggesting successful uptake of paclitaxel by vessel walls after DEB dilation.
Conclusions: The novel DEB is safe and has a favorable vascular healing response on neointimal hyperplasia.
Keywords: Neointimal hyperplasia; paclitaxel-eluting balloon; pharmacokinetics.
Figures



Similar articles
-
Optical coherence tomography (OCT) in PCI for in-stent restenosis (ISR): rationale and design of the SEDUCE (Safety and Efficacy of a Drug elUting balloon in Coronary artery rEstenosis) study.EuroIntervention. 2011 May;7 Suppl K:K100-5. doi: 10.4244/EIJV7SKA17. EuroIntervention. 2011. PMID: 22027717 Clinical Trial.
-
Stent coverage and neointimal proliferation in bare metal stents postdilated with a Paclitaxel-eluting balloon versus everolimus-eluting stents: prospective randomized study using optical coherence tomography at 6-month follow-up.Circ Cardiovasc Interv. 2014 Dec;7(6):760-7. doi: 10.1161/CIRCINTERVENTIONS.113.001146. Epub 2014 Nov 4. Circ Cardiovasc Interv. 2014. PMID: 25371536 Clinical Trial.
-
Optical coherence tomography study of healing characteristics of paclitaxel-eluting balloons vs. everolimus-eluting stents for in-stent restenosis: the SEDUCE (Safety and Efficacy of a Drug elUting balloon in Coronary artery rEstenosis) randomised clinical trial.EuroIntervention. 2014 Aug;10(4):439-48. doi: 10.4244/EIJV10I4A77. EuroIntervention. 2014. PMID: 25138182 Clinical Trial.
-
Drug-eluting balloons in coronary interventions: the quiet revolution?Expert Opin Drug Deliv. 2017 Jul;14(7):841-850. doi: 10.1080/17425247.2017.1245291. Epub 2016 Oct 19. Expert Opin Drug Deliv. 2017. PMID: 27718756 Review.
-
Drug-Eluting Balloons in the Treatment of Coronary De Novo Lesions: A Comprehensive Review.Cardiol Ther. 2016 Dec;5(2):133-160. doi: 10.1007/s40119-016-0064-4. Epub 2016 Jul 6. Cardiol Ther. 2016. PMID: 27384194 Free PMC article. Review.
Cited by
-
Reduced histologic neo in-stent restenosis after use of a paclitaxel-coated cutting balloon in porcine coronary arteries.Histol Histopathol. 2020 Jul;35(7):653-663. doi: 10.14670/HH-18-177. Epub 2019 Oct 24. Histol Histopathol. 2020. PMID: 31646547
-
Techniques in Vascular and Interventional Radiology Drug Delivery Technologies in the Superficial Femoral Artery.Tech Vasc Interv Radiol. 2016 Jun;19(2):145-52. doi: 10.1053/j.tvir.2016.04.007. Epub 2016 May 5. Tech Vasc Interv Radiol. 2016. PMID: 27423996 Free PMC article.
-
Drug-coated balloon angioplasty with provisional stenting versus primary stenting for the treatment of de novo coronary artery lesions: REC-CAGEFREE I trial rationale and design.BMC Cardiovasc Disord. 2024 Jun 24;24(1):319. doi: 10.1186/s12872-024-03974-0. BMC Cardiovasc Disord. 2024. PMID: 38914951 Free PMC article.
References
-
- R. Ross, Atherosclerosis – An inflammatory disease, N. Engl. J. Med. 340(2) (1999), 115–126. - PubMed
-
- M.C. Morice, P.W. Serruys, J.E. Sousa, J. Fajadet, E. Ban Hayashi, M. Perin et al., A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization, N. Engl. J. Med. 346(23) (2002), 1773–1780. - PubMed
-
- H.J. Rapold, P.R. David, P. Guiteras Val, A.L. Mata, P.A. Crean and M.G. Bourassa, Restenosis and its determinants in first and repeat coronary angioplasty, Eur. Heart J. 8(6) (1987), 575–586. - PubMed
-
- P.V. Serruys, P. de Jaegere, F. Kiemeneij, C. Macaya, W. Rutsch, G. Heyndrickx et al., A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent study group, N. Engl. J. Med. 331(8) (1994), 489–495. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

