A Novel Approach to High-Quality Postmortem Tissue Procurement: The GTEx Project
- PMID: 26484571
- PMCID: PMC4675181
- DOI: 10.1089/bio.2015.0032
A Novel Approach to High-Quality Postmortem Tissue Procurement: The GTEx Project
Abstract
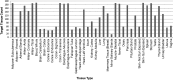
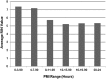
The Genotype-Tissue Expression (GTEx) project, sponsored by the NIH Common Fund, was established to study the correlation between human genetic variation and tissue-specific gene expression in non-diseased individuals. A significant challenge was the collection of high-quality biospecimens for extensive genomic analyses. Here we describe how a successful infrastructure for biospecimen procurement was developed and implemented by multiple research partners to support the prospective collection, annotation, and distribution of blood, tissues, and cell lines for the GTEx project. Other research projects can follow this model and form beneficial partnerships with rapid autopsy and organ procurement organizations to collect high quality biospecimens and associated clinical data for genomic studies. Biospecimens, clinical and genomic data, and Standard Operating Procedures guiding biospecimen collection for the GTEx project are available to the research community.
Figures




Comment in
-
The Genotype-Tissue Expression (GTEx) Project.Biopreserv Biobank. 2015 Oct;13(5):307-8. doi: 10.1089/bio.2015.29031.hmm. Biopreserv Biobank. 2015. PMID: 26484569 Free PMC article. No abstract available.
References
-
- Mucci NR, Moore H, Brigham LE, et al. Meeting research needs with postmortem biospecimen donation: Summary of recommendations for postmortem recovery of normal human biospecimens for research. Biopreserv Biobank 2013;11:77–82 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

