Rescue of a Plant Negative-Strand RNA Virus from Cloned cDNA: Insights into Enveloped Plant Virus Movement and Morphogenesis
- PMID: 26484673
- PMCID: PMC4616665
- DOI: 10.1371/journal.ppat.1005223
Rescue of a Plant Negative-Strand RNA Virus from Cloned cDNA: Insights into Enveloped Plant Virus Movement and Morphogenesis
Abstract
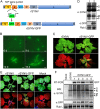
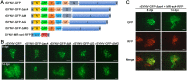
Reverse genetics systems have been established for all major groups of plant DNA and positive-strand RNA viruses, and our understanding of their infection cycles and pathogenesis has benefitted enormously from use of these approaches. However, technical difficulties have heretofore hampered applications of reverse genetics to plant negative-strand RNA (NSR) viruses. Here, we report recovery of infectious virus from cloned cDNAs of a model plant NSR, Sonchus yellow net rhabdovirus (SYNV). The procedure involves Agrobacterium-mediated transcription of full-length SYNV antigenomic RNA and co-expression of the nucleoprotein (N), phosphoprotein (P), large polymerase core proteins and viral suppressors of RNA silencing in Nicotiana benthamiana plants. Optimization of core protein expression resulted in up to 26% recombinant SYNV (rSYNV) infections of agroinfiltrated plants. A reporter virus, rSYNV-GFP, engineered by inserting a green fluorescence protein (GFP) gene between the N and P genes was able to express GFP during systemic infections and after repeated plant-to-plant mechanical passages. Deletion analyses with rSYNV-GFP demonstrated that SYNV cell-to-cell movement requires the sc4 protein and suggested that uncoiled nucleocapsids are infectious movement entities. Deletion analyses also showed that the glycoprotein is not required for systemic infection, although the glycoprotein mutant was defective in virion morphogenesis. Taken together, we have developed a robust reverse genetics system for SYNV that provides key insights into morphogenesis and movement of an enveloped plant virus. Our study also provides a template for developing analogous systems for reverse genetic analysis of other plant NSR viruses.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Construction of a Sonchus Yellow Net Virus minireplicon: a step toward reverse genetic analysis of plant negative-strand RNA viruses.J Virol. 2013 Oct;87(19):10598-611. doi: 10.1128/JVI.01397-13. Epub 2013 Jul 24. J Virol. 2013. PMID: 23885070 Free PMC article.
-
Significantly Improved Recovery of Recombinant Sonchus Yellow Net Rhabdovirus by Expressing the Negative-Strand Genomic RNA.Viruses. 2020 Dec 17;12(12):1459. doi: 10.3390/v12121459. Viruses. 2020. PMID: 33348798 Free PMC article.
-
Capped antigenomic RNA transcript facilitates rescue of a plant rhabdovirus.Virol J. 2017 Jun 13;14(1):113. doi: 10.1186/s12985-017-0776-7. Virol J. 2017. PMID: 28610585 Free PMC article.
-
Developments in Plant Negative-Strand RNA Virus Reverse Genetics.Annu Rev Phytopathol. 2016 Aug 4;54:469-98. doi: 10.1146/annurev-phyto-080615-095909. Epub 2016 Jan 17. Annu Rev Phytopathol. 2016. PMID: 27359368 Review.
-
Development of Model Systems for Plant Rhabdovirus Research.Adv Virus Res. 2018;102:23-57. doi: 10.1016/bs.aivir.2018.06.008. Epub 2018 Jul 30. Adv Virus Res. 2018. PMID: 30266175 Review.
Cited by
-
Two Novel Rhabdoviruses Related to Hypervirulence in a Phytopathogenic Fungus.J Virol. 2022 Apr 27;96(8):e0001222. doi: 10.1128/jvi.00012-22. Epub 2022 Apr 7. J Virol. 2022. PMID: 35389267 Free PMC article.
-
Developing reverse genetics systems of northern cereal mosaic virus to reveal superinfection exclusion of two cytorhabdoviruses in barley plants.Mol Plant Pathol. 2022 May;23(5):749-756. doi: 10.1111/mpp.13188. Epub 2022 Feb 6. Mol Plant Pathol. 2022. PMID: 35124878 Free PMC article.
-
Transgene-free Genome Editing in Plants.Front Genome Ed. 2021 Dec 2;3:805317. doi: 10.3389/fgeed.2021.805317. eCollection 2021. Front Genome Ed. 2021. PMID: 34927134 Free PMC article. Review.
-
Specificity of Plant Rhabdovirus Cell-to-Cell Movement.J Virol. 2019 Jul 17;93(15):e00296-19. doi: 10.1128/JVI.00296-19. Print 2019 Aug 1. J Virol. 2019. PMID: 31118256 Free PMC article.
-
Development of a minigenome cassette for Lettuce necrotic yellows virus: A first step in rescuing a plant cytorhabdovirus.PLoS One. 2020 Mar 5;15(3):e0229877. doi: 10.1371/journal.pone.0229877. eCollection 2020. PLoS One. 2020. PMID: 32134974 Free PMC article.
References
-
- King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses. London, UK: Elsevier Academic Press; 2011.
-
- Falk BW, Tsai JH. Biology and molecular biology of viruses in the genus Tenuivirus. Annu Rev Phytopathol. 1998; 36: 139–163. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

