Effects of Topical Bimatoprost 0.01% and Timolol 0.5% on Circadian IOP, Blood Pressure and Perfusion Pressure in Patients with Glaucoma or Ocular Hypertension: A Randomized, Double Masked, Placebo-Controlled Clinical Trial
- PMID: 26484767
- PMCID: PMC4615626
- DOI: 10.1371/journal.pone.0140601
Effects of Topical Bimatoprost 0.01% and Timolol 0.5% on Circadian IOP, Blood Pressure and Perfusion Pressure in Patients with Glaucoma or Ocular Hypertension: A Randomized, Double Masked, Placebo-Controlled Clinical Trial
Abstract
Purpose: To compare the 24-hour (24h) effects on intraocular pressure (IOP) and cardiovascular parameters of timolol 0.5% and bimatoprost 0.01% in open angle glaucoma and ocular hypertensive subjects.
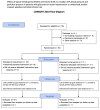
Methods: In this prospective, randomized, double masked, crossover, clinical trial, after washout from previous medications enrolled subjects underwent 24h IOP, blood pressure (BP) and heart rate (HR) measurements and were randomized to either topical bimatoprost 0.01% at night plus placebo in the morning or to timolol 0.5% bid. After 8 weeks of treatment a second 24h assessment of IOP, BP and HR was performed and then subjects switched to the opposite treatment for additional 8 weeks when a third 24h assessment was performed. The primary endpoint was the comparison of the mean 24h IOP after each treatment. Secondary endpoints included the comparisons of IOP at each timepoint of the 24h curve and the comparison of BP, HR, ocular perfusion pressure and tolerability.
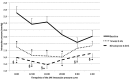
Results: Mean untreated 24h IOP was 20.3 mmHg (95%CI 19.0 to 21.6). Mean 24h IOP was significantly lower after 8 weeks of treatment with bimatoprost 0.01% than after 8 weeks of treatment with timolol 0.5% bid (15.7 vs 16.8 mmHg, p = 0.0003). Mean IOP during the day hours was significantly reduced from baseline by both drugs while mean IOP during the night hours was reduced by -2.3 mmHg (p = 0.0002) by bimatoprost 0.01% plus placebo and by -1.1 mmHg by timolol 0.5% bid (p = 0.06). Timolol 0.5% significantly reduced the mean 24h systolic BP from baseline, the diastolic BP during the day hours, the HR during the night hours, and the mean 24h systolic ocular perfusion pressure.
Conclusion: Both Bimatoprost 0.01% and Timolol 0.5% are effective in reducing the mean 24h IOP from an untreated baseline but Bimatoprost 0.01% is more effective than timolol 0.5% throughout the 24h. Timolol 0.5% effect on IOP is reduced during the night hours and is associated with reduced BP, HR and ocular perfusion pressure.
Trial registration: EU Clinical Trial Register and EudraCT# 2010-024272-26.
Conflict of interest statement
Figures


Similar articles
-
Comparison of the effects of bimatoprost and a fixed combination of latanoprost and timolol on circadian intraocular pressure.Ophthalmology. 2007 Dec;114(12):2244-51. doi: 10.1016/j.ophtha.2007.01.025. Epub 2007 Apr 25. Ophthalmology. 2007. PMID: 17459480 Clinical Trial.
-
Efficacy and tolerability of fixed-combination bimatoprost/timolol versus fixed-combination dorzolamide/brimonidine/timolol in patients with primary open-angle glaucoma or ocular hypertension: a multicenter, prospective, crossover study.BMC Ophthalmol. 2014 Dec 19;14:161. doi: 10.1186/1471-2415-14-161. BMC Ophthalmol. 2014. PMID: 25527295 Free PMC article. Clinical Trial.
-
Six-Month Intraocular Pressure Reduction with a Topical Bimatoprost Ocular Insert: Results of a Phase II Randomized Controlled Study.Ophthalmology. 2016 Aug;123(8):1685-1694. doi: 10.1016/j.ophtha.2016.04.026. Epub 2016 May 5. Ophthalmology. 2016. PMID: 27157843 Clinical Trial.
-
Intraocular pressure-lowering efficacy and ocular safety of Rho-kinase inhibitor in glaucoma: a meta-analysis and systematic review of prospective randomized trials.Graefes Arch Clin Exp Ophthalmol. 2022 Mar;260(3):937-948. doi: 10.1007/s00417-021-05379-7. Epub 2021 Sep 7. Graefes Arch Clin Exp Ophthalmol. 2022. PMID: 34491427
-
Topical medical therapy and ocular perfusion pressure in open angle glaucoma: a systematic review and meta-analysis.Curr Med Res Opin. 2019 Aug;35(8):1421-1431. doi: 10.1080/03007995.2019.1595553. Epub 2019 Apr 29. Curr Med Res Opin. 2019. PMID: 30880485
Cited by
-
Effects of timolol maleate eye drops on experimentally dilated cardiomyopathy and healthy rabbits.Open Vet J. 2021 Jul-Sep;11(3):390-393. doi: 10.5455/OVJ.2021.v11.i3.9. Epub 2021 Aug 5. Open Vet J. 2021. PMID: 34722201 Free PMC article.
-
Magnesium Hydroxide Nanoparticles Improve the Ocular Hypotensive Effect of Twice Daily Topical Timolol Maleate in Healthy Dogs.Vet Sci. 2021 Aug 23;8(8):168. doi: 10.3390/vetsci8080168. Vet Sci. 2021. PMID: 34437490 Free PMC article.
-
Sustained Release Therapies with the Prostaglandin Analogues Intracameral Implants.Ophthalmol Ther. 2024 Jul;13(7):1833-1839. doi: 10.1007/s40123-024-00965-4. Epub 2024 May 18. Ophthalmol Ther. 2024. PMID: 38761359 Free PMC article.
-
The 24-Hour Effects of Brinzolamide/Brimonidine Fixed Combination and Timolol on Intraocular Pressure and Ocular Perfusion Pressure.J Ocul Pharmacol Ther. 2017 Apr;33(3):161-169. doi: 10.1089/jop.2016.0141. Epub 2017 Jan 27. J Ocul Pharmacol Ther. 2017. PMID: 28129020 Free PMC article. Clinical Trial.
-
Twenty-Four-Hour Intraocular Pressure Control with Omidenepag Isopropyl 0.002% in Patients with Glaucoma and Ocular Hypertension.Clin Ophthalmol. 2021 Oct 4;15:3997-4003. doi: 10.2147/OPTH.S333042. eCollection 2021. Clin Ophthalmol. 2021. PMID: 34675468 Free PMC article.
References
-
- Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E. Factors for Glaucoma progression and the effect of treatment: the Early Manifest Glaucoma Trial. Arch Ophthalmol 2003;121:48–56. - PubMed
-
- The AGIS Investigators. Advanced Glaucoma Intervention Study. 7. The relationship between control of intraocular pressure and visual field deterioration. AM J Ophthalmol 2000;130:429–40. - PubMed
-
- NICE. Published clinical guidelines CG85 Glaucoma. 1st edn. London: National Institute of Health and Clinical Excellence; 2009.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

