Diffusion MRI in the heart
- PMID: 26484848
- PMCID: PMC5333463
- DOI: 10.1002/nbm.3426
Diffusion MRI in the heart
Abstract
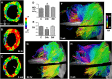
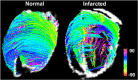
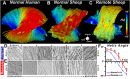
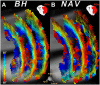
Diffusion MRI provides unique information on the structure, organization, and integrity of the myocardium without the need for exogenous contrast agents. Diffusion MRI in the heart, however, has proven technically challenging because of the intrinsic non-rigid deformation during the cardiac cycle, displacement of the myocardium due to respiratory motion, signal inhomogeneity within the thorax, and short transverse relaxation times. Recently developed accelerated diffusion-weighted MR acquisition sequences combined with advanced post-processing techniques have improved the accuracy and efficiency of diffusion MRI in the myocardium. In this review, we describe the solutions and approaches that have been developed to enable diffusion MRI of the heart in vivo, including a dual-gated stimulated echo approach, a velocity- (M1 ) or an acceleration- (M2 ) compensated pulsed gradient spin echo approach, and the use of principal component analysis filtering. The structure of the myocardium and the application of these techniques in ischemic heart disease are also briefly reviewed. The advent of clinical MR systems with stronger gradients will likely facilitate the translation of cardiac diffusion MRI into clinical use. The addition of diffusion MRI to the well-established set of cardiovascular imaging techniques should lead to new and complementary approaches for the diagnosis and evaluation of patients with heart disease. © 2015 The Authors. NMR in Biomedicine published by John Wiley & Sons Ltd.
Keywords: MRI; diffusion; heart; ischemia; myocardium; myofiber architecture; tensor; tractography.
© 2015 The Authors. NMR in Biomedicine published by John Wiley & Sons Ltd.
Figures



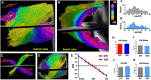
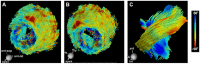

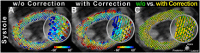

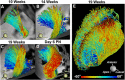


References
-
- Edelman RR, Gaa J, Wedeen VJ, Loh E, Hare JM, Prasad P, Li W. In vivo measurement of water diffusion in the human heart. Magn. Reson. Med. 1994; 32(3): 423–428. - PubMed
-
- Reese TG, Wedeen VJ, Weisskoff RM. Measuring diffusion in the presence of material strain. J. Magn. Reson. B 1996; 112(3): 253–258. - PubMed
-
- Reese TG, Weisskoff RM, Smith RN, Rosen BR, Dinsmore RE, Wedeen VJ. Imaging myocardial fiber architecture in vivo with magnetic resonance. Magn. Reson. Med. 1995; 34(6): 786–791. - PubMed
-
- van Heeswijk RB, Feliciano H, Bongard C, Bonanno G, Coppo S, Lauriers N, Locca D, Schwitter J, Stuber M. Free‐breathing 3 T magnetic resonance T2‐mapping of the heart. JACC Cardiovasc. Imaging 2012; 5(12): 1231–1239. - PubMed
-
- van Heeswijk RB, Piccini D, Feliciano H, Hullin R, Schwitter J, Stuber M. Self‐navigated isotropic three‐dimensional cardiac T2 mapping. Magn. Reson. Med. 2015; 73(4): 1549–1554. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

