At the Edge of Chaos: How Cerebellar Granular Layer Network Dynamics Can Provide the Basis for Temporal Filters
- PMID: 26484859
- PMCID: PMC4615637
- DOI: 10.1371/journal.pcbi.1004515
At the Edge of Chaos: How Cerebellar Granular Layer Network Dynamics Can Provide the Basis for Temporal Filters
Abstract
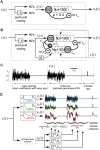
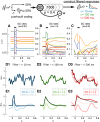
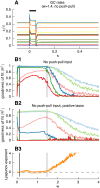
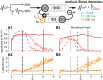
Models of the cerebellar microcircuit often assume that input signals from the mossy-fibers are expanded and recoded to provide a foundation from which the Purkinje cells can synthesize output filters to implement specific input-signal transformations. Details of this process are however unclear. While previous work has shown that recurrent granule cell inhibition could in principle generate a wide variety of random outputs suitable for coding signal onsets, the more general application for temporally varying signals has yet to be demonstrated. Here we show for the first time that using a mechanism very similar to reservoir computing enables random neuronal networks in the granule cell layer to provide the necessary signal separation and extension from which Purkinje cells could construct basis filters of various time-constants. The main requirement for this is that the network operates in a state of criticality close to the edge of random chaotic behavior. We further show that the lack of recurrent excitation in the granular layer as commonly required in traditional reservoir networks can be circumvented by considering other inherent granular layer features such as inverted input signals or mGluR2 inhibition of Golgi cells. Other properties that facilitate filter construction are direct mossy fiber excitation of Golgi cells, variability of synaptic weights or input signals and output-feedback via the nucleocortical pathway. Our findings are well supported by previous experimental and theoretical work and will help to bridge the gap between system-level models and detailed models of the granular layer network.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Synchronization of golgi and granule cell firing in a detailed network model of the cerebellar granule cell layer.J Neurophysiol. 1998 Nov;80(5):2521-37. doi: 10.1152/jn.1998.80.5.2521. J Neurophysiol. 1998. PMID: 9819260
-
Chaos may enhance expressivity in cerebellar granular layer.Neural Netw. 2021 Apr;136:72-86. doi: 10.1016/j.neunet.2020.12.020. Epub 2020 Dec 28. Neural Netw. 2021. PMID: 33450654
-
Spatiotemporal network coding of physiological mossy fiber inputs by the cerebellar granular layer.PLoS Comput Biol. 2017 Sep 21;13(9):e1005754. doi: 10.1371/journal.pcbi.1005754. eCollection 2017 Sep. PLoS Comput Biol. 2017. PMID: 28934196 Free PMC article.
-
Timing and plasticity in the cerebellum: focus on the granular layer.Trends Neurosci. 2009 Jan;32(1):30-40. doi: 10.1016/j.tins.2008.09.007. Epub 2008 Oct 30. Trends Neurosci. 2009. PMID: 18977038 Review.
-
The criticality hypothesis: how local cortical networks might optimize information processing.Philos Trans A Math Phys Eng Sci. 2008 Feb 13;366(1864):329-43. doi: 10.1098/rsta.2007.2092. Philos Trans A Math Phys Eng Sci. 2008. PMID: 17673410 Review.
Cited by
-
Neonatal Hyperoxia Perturbs Neuronal Development in the Cerebellum.Mol Neurobiol. 2018 May;55(5):3901-3915. doi: 10.1007/s12035-017-0612-5. Epub 2017 May 25. Mol Neurobiol. 2018. PMID: 28547531
-
A cerebellum inspired spiking neural network as a multi-model for pattern classification and robotic trajectory prediction.Front Neurosci. 2022 Nov 28;16:909146. doi: 10.3389/fnins.2022.909146. eCollection 2022. Front Neurosci. 2022. PMID: 36518530 Free PMC article.
-
Motor context dominates output from purkinje cell functional regions during reflexive visuomotor behaviours.Elife. 2019 Jan 25;8:e42138. doi: 10.7554/eLife.42138. Elife. 2019. PMID: 30681408 Free PMC article.
-
Introducing chaotic codes for the modulation of code modulated visual evoked potentials (c-VEP) in normal adults for visual fatigue reduction.PLoS One. 2019 Mar 6;14(3):e0213197. doi: 10.1371/journal.pone.0213197. eCollection 2019. PLoS One. 2019. PMID: 30840671 Free PMC article.
-
Parameter tuning differentiates granule cell subtypes enriching transmission properties at the cerebellum input stage.Commun Biol. 2020 May 8;3(1):222. doi: 10.1038/s42003-020-0953-x. Commun Biol. 2020. PMID: 32385389 Free PMC article.
References
-
- Albus JS. A theory of cerebellar function. Math Biosci. 1971;10: 25–61.
-
- Fujita M. Adaptive filter model of the cerebellum. Biol Cybern. 1982;45: 195–206. - PubMed
-
- Dean DP, Jörntell DH, Porrill DJ. Adaptive Filter Models In: Manto M, Schmahmann JD, Rossi F, Gruol DL, Koibuchi N, editors. Handbook of the Cerebellum and Cerebellar Disorders. Springer; Netherlands; 2013. pp. 1315–1335. Available: http://link.springer.com/referenceworkentry/10.1007/978-94-007-1333-8_58 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

