Protein Homeostasis Imposes a Barrier on Functional Integration of Horizontally Transferred Genes in Bacteria
- PMID: 26484862
- PMCID: PMC4618355
- DOI: 10.1371/journal.pgen.1005612
Protein Homeostasis Imposes a Barrier on Functional Integration of Horizontally Transferred Genes in Bacteria
Abstract
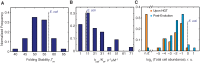
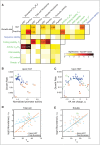
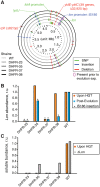
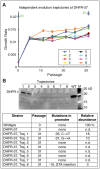
Horizontal gene transfer (HGT) plays a central role in bacterial evolution, yet the molecular and cellular constraints on functional integration of the foreign genes are poorly understood. Here we performed inter-species replacement of the chromosomal folA gene, encoding an essential metabolic enzyme dihydrofolate reductase (DHFR), with orthologs from 35 other mesophilic bacteria. The orthologous inter-species replacements caused a marked drop (in the range 10-90%) in bacterial growth rate despite the fact that most orthologous DHFRs are as stable as E.coli DHFR at 37°C and are more catalytically active than E. coli DHFR. Although phylogenetic distance between E. coli and orthologous DHFRs as well as their individual molecular properties correlate poorly with growth rates, the product of the intracellular DHFR abundance and catalytic activity (kcat/KM), correlates strongly with growth rates, indicating that the drop in DHFR abundance constitutes the major fitness barrier to HGT. Serial propagation of the orthologous strains for ~600 generations dramatically improved growth rates by largely alleviating the fitness barriers. Whole genome sequencing and global proteome quantification revealed that the evolved strains with the largest fitness improvements have accumulated mutations that inactivated the ATP-dependent Lon protease, causing an increase in the intracellular DHFR abundance. In one case DHFR abundance increased further due to mutations accumulated in folA promoter, but only after the lon inactivating mutations were fixed in the population. Thus, by apparently distinguishing between self and non-self proteins, protein homeostasis imposes an immediate and global barrier to the functional integration of foreign genes by decreasing the intracellular abundance of their products. Once this barrier is alleviated, more fine-tuned evolution occurs to adjust the function/expression of the transferred proteins to the constraints imposed by the intracellular environment of the host organism.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Gogarten JP, Townsend JP (2005) Horizontal gene transfer, genome innovation and evolution. Nat Rev Microbiol 3: 679–687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

