Increased Sensitivity to Binge Alcohol-Induced Gut Leakiness and Inflammatory Liver Disease in HIV Transgenic Rats
- PMID: 26484872
- PMCID: PMC4618849
- DOI: 10.1371/journal.pone.0140498
Increased Sensitivity to Binge Alcohol-Induced Gut Leakiness and Inflammatory Liver Disease in HIV Transgenic Rats
Abstract
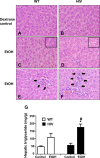
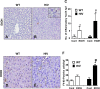
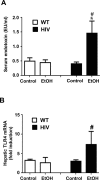
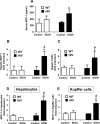
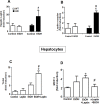
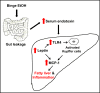
The mechanisms of alcohol-mediated advanced liver injury in HIV-infected individuals are poorly understood. Thus, this study was aimed to investigate the effect of binge alcohol on the inflammatory liver disease in HIV transgenic rats as a model for simulating human conditions. Female wild-type (WT) or HIV transgenic rats were treated with three consecutive doses of binge ethanol (EtOH) (3.5 g/kg/dose oral gavages at 12-h intervals) or dextrose (Control). Blood and liver tissues were collected at 1 or 6-h following the last dose of ethanol or dextrose for the measurements of serum endotoxin and liver pathology, respectively. Compared to the WT, the HIV rats showed increased sensitivity to alcohol-mediated gut leakiness, hepatic steatosis and inflammation, as evidenced with the significantly elevated levels of serum endotoxin, hepatic triglycerides, histological fat accumulation and F4/80 staining. Real-time PCR analysis revealed that hepatic levels of toll-like receptor-4 (TLR4), leptin and the downstream target monocyte chemoattractant protein-1 (MCP-1) were significantly up-regulated in the HIV-EtOH rats, compared to all other groups. Subsequent experiments with primary cultured cells showed that both hepatocytes and hepatic Kupffer cells were the sources of the elevated MCP-1 in HIV-EtOH rats. Further, TLR4 and MCP-1 were found to be upregulated by leptin. Collectively, these results show that HIV rats, similar to HIV-infected people being treated with the highly active anti-retroviral therapy (HAART), are more susceptible to binge alcohol-induced gut leakiness and inflammatory liver disease than the corresponding WT, possibly due to additive or synergistic interaction between binge alcohol exposure and HIV infection. Based on these results, HIV transgenic rats can be used as a surrogate model to study the molecular mechanisms of many disease states caused by heavy alcohol intake in HIV-infected people on HAART.
Conflict of interest statement
Figures
Similar articles
-
5-ALA/SFC Attenuated Binge Alcohol-Induced Gut Leakiness and Inflammatory Liver Disease in HIV Transgenic Rats.Alcohol Clin Exp Res. 2019 Aug;43(8):1651-1661. doi: 10.1111/acer.14117. Epub 2019 Jun 13. Alcohol Clin Exp Res. 2019. PMID: 31141180
-
CYP2E1 potentiates binge alcohol-induced gut leakiness, steatohepatitis, and apoptosis.Free Radic Biol Med. 2013 Dec;65:1238-1245. doi: 10.1016/j.freeradbiomed.2013.09.009. Epub 2013 Sep 21. Free Radic Biol Med. 2013. PMID: 24064383 Free PMC article.
-
Pomegranate prevents binge alcohol-induced gut leakiness and hepatic inflammation by suppressing oxidative and nitrative stress.Redox Biol. 2018 Sep;18:266-278. doi: 10.1016/j.redox.2018.07.012. Epub 2018 Jul 20. Redox Biol. 2018. PMID: 30071471 Free PMC article.
-
Circadian rhythms, alcohol and gut interactions.Alcohol. 2015 Jun;49(4):389-98. doi: 10.1016/j.alcohol.2014.07.021. Epub 2014 Nov 14. Alcohol. 2015. PMID: 25499101 Free PMC article. Review.
-
Rodent Models of Alcoholic Liver Disease: Role of Binge Ethanol Administration.Biomolecules. 2018 Jan 13;8(1):3. doi: 10.3390/biom8010003. Biomolecules. 2018. PMID: 29342874 Free PMC article. Review.
Cited by
-
Macrophage Migration Inhibitor Factor Upregulates MCP-1 Expression in an Autocrine Manner in Hepatocytes during Acute Mouse Liver Injury.Sci Rep. 2016 Jun 8;6:27665. doi: 10.1038/srep27665. Sci Rep. 2016. PMID: 27273604 Free PMC article.
-
Alcohol Use and Abuse Conspires With HIV Infection to Aggravate Intestinal Dysbiosis and Increase Microbial Translocation in People Living With HIV: A Review.Front Immunol. 2021 Dec 17;12:741658. doi: 10.3389/fimmu.2021.741658. eCollection 2021. Front Immunol. 2021. PMID: 34975838 Free PMC article. Review.
-
Targeting of CYP2E1 by miRNAs in alcohol-induced intestine injury.Mol Cells. 2024 Jul;47(7):100074. doi: 10.1016/j.mocell.2024.100074. Epub 2024 Jun 18. Mol Cells. 2024. PMID: 38901530 Free PMC article.
-
Preventive effects of indole-3-carbinol against alcohol-induced liver injury in mice via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms: Role of gut-liver-adipose tissue axis.J Nutr Biochem. 2018 May;55:12-25. doi: 10.1016/j.jnutbio.2017.11.011. Epub 2017 Dec 10. J Nutr Biochem. 2018. PMID: 29331880 Free PMC article.
-
Effects of alcohol on the composition and metabolism of the intestinal microbiota among people with HIV: A cross-sectional study.Alcohol. 2024 Nov;120:151-159. doi: 10.1016/j.alcohol.2024.02.003. Epub 2024 Feb 20. Alcohol. 2024. PMID: 38387693 Free PMC article.
References
-
- Center for Disease Control (CDC), Division of HIV/AIDS Prevention. HIV in the United States. 2011.
-
- Petry NM (1999) Alcohol use in HIV patients: what we don’t know may hurt us. Int J Std AIDS 10: 561–570. - PubMed
-
- Conigliaro J, Gordon AJ, McGinnis KA, Rabeneck L, Justice AC, Veterans Aging Cohort 3-Site Study (2003) How harmful is hazadous alcohol use and abuse in HIV infection: do health care provides know who is at risk? J Acquir Immune Defic Syndr 33: 521–525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

