Novel Types of Small RNA Exhibit Sequence- and Target-dependent Angiogenesis Suppression Without Activation of Toll-like Receptor 3 in an Age-related Macular Degeneration (AMD) Mouse Model
- PMID: 26484944
- PMCID: PMC4881762
- DOI: 10.1038/mtna.2015.34
Novel Types of Small RNA Exhibit Sequence- and Target-dependent Angiogenesis Suppression Without Activation of Toll-like Receptor 3 in an Age-related Macular Degeneration (AMD) Mouse Model
Abstract
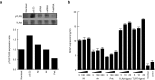
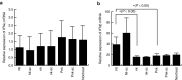
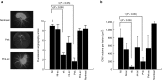
RNA interference (RNAi) has become a powerful tool for suppressing gene expression in vitro and in vivo. A great deal of evidence has demonstrated the potential for the use of synthetic small interfering RNAs (siRNAs) as therapeutic agents. However, the application of siRNA to clinical medicine is still limited, mainly due to sequence-independent suppression of angiogenesis mediated by Toll-like receptor 3 (TLR3). Here, we describe novel types of synthetic RNA, named nkRNA and PnkRNA, that exhibit sequence-specific gene silencing through RNAi without activating TLRs or RIG-I-like receptor signaling. In addition, we confirmed the therapeutic effect for the novel types of RNA in an animal model of age-related macular degeneration (AMD) without retinal degeneration. These data indicate that nkRNA and PnkRNA are of great potential utility as therapies against blinding choroidal neovascularization due to AMD.
Figures


Similar articles
-
Sequence- and target-independent angiogenesis suppression by siRNA via TLR3.Nature. 2008 Apr 3;452(7187):591-7. doi: 10.1038/nature06765. Epub 2008 Mar 26. Nature. 2008. PMID: 18368052 Free PMC article.
-
Short-interfering RNAs induce retinal degeneration via TLR3 and IRF3.Mol Ther. 2012 Jan;20(1):101-8. doi: 10.1038/mt.2011.212. Epub 2011 Oct 11. Mol Ther. 2012. PMID: 21988875 Free PMC article.
-
Development of gene therapy for treatment of age-related macular degeneration.Acta Ophthalmol. 2014 Jul;92 Thesis3:1-38. doi: 10.1111/aos.12452. Acta Ophthalmol. 2014. PMID: 24953666
-
[Novel approach for management of age-related macular degeneration--antiangiogenic therapy and retinal regenerative therapy].Nippon Ganka Gakkai Zasshi. 2007 Mar;111(3):232-68; discussion 269. Nippon Ganka Gakkai Zasshi. 2007. PMID: 17402564 Review. Japanese.
-
Technology of RNA Interference in Advanced Medicine.Microrna. 2018;7(2):74-84. doi: 10.2174/2211536607666180129153307. Microrna. 2018. PMID: 29380708 Review.
Cited by
-
A Small Indel Mutant Mouse Model of Epidermolytic Palmoplantar Keratoderma and Its Application to Mutant-specific shRNA Therapy.Mol Ther Nucleic Acids. 2016 Mar 22;5(3):e299. doi: 10.1038/mtna.2016.17. Mol Ther Nucleic Acids. 2016. PMID: 27003758 Free PMC article.
-
Topical Use of Angiopoietin-like Protein 2 RNAi-loaded Lipid Nanoparticles Suppresses Corneal Neovascularization.Mol Ther Nucleic Acids. 2016 Mar 8;5(3):e292. doi: 10.1038/mtna.2016.1. Mol Ther Nucleic Acids. 2016. PMID: 27111418 Free PMC article.
-
PKR promotes choroidal neovascularization via upregulating the PI3K/Akt signaling pathway in VEGF expression.Mol Vis. 2016 Dec 2;22:1361-1374. eCollection 2016. Mol Vis. 2016. PMID: 27994435 Free PMC article.
-
Novel form of miR-29b suppresses bleomycin-induced pulmonary fibrosis.PLoS One. 2017 Feb 24;12(2):e0171957. doi: 10.1371/journal.pone.0171957. eCollection 2017. PLoS One. 2017. PMID: 28234907 Free PMC article.
-
Periostin in vitreoretinal diseases.Cell Mol Life Sci. 2017 Dec;74(23):4329-4337. doi: 10.1007/s00018-017-2651-5. Epub 2017 Sep 14. Cell Mol Life Sci. 2017. PMID: 28913545 Free PMC article. Review.
References
-
- Waisbourd, M, Loewenstein, A, Goldstein, M and Leibovitch, I (2007). Targeting vascular endothelial growth factor: a promising strategy for treating age-related macular degeneration. Drugs Aging 24: 643–662. - PubMed
-
- Alexopoulou, L, Holt, AC, Medzhitov, R and Flavell, RA (2001). Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413: 732–738. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

