Advances in the Diagnosis of Human Opisthorchiasis: Development of Opisthorchis viverrini Antigen Detection in Urine
- PMID: 26485024
- PMCID: PMC4618926
- DOI: 10.1371/journal.pntd.0004157
Advances in the Diagnosis of Human Opisthorchiasis: Development of Opisthorchis viverrini Antigen Detection in Urine
Abstract
Background: Many strategies to control opisthorchiasis have been employed in Thailand, but not in the other neighbouring countries. Specific control methods include mass drug administration (MDA) and health education to reduce raw fish consumption. These control efforts have greatly shifted the epidemiology of Opisthorchis viverrini (OV) infection over the last decade from presenting as densely concentrated "heavy" infections in single villages to widespread "light" OV infections distributed over wide geographical areas. Currently, the "gold standard" detection method for OV infection is formalin ethyl-acetate concentration technique (FECT), which has limited diagnostic sensitivity and diagnostic specificity for light OV infections, with OV eggs often confused with eggs of minute intestinal flukes (MIFs) in feces. In this study, we developed and evaluated the diagnostic performance of a monoclonal antibody-based enzyme-linked immunosorbent assay for the measurement of OV excretory-secretory (ES) antigens in urine (urine OV-ES assay) for the diagnosis of opisthorchiasis compared to the gold standard detection FECT method.
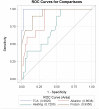
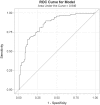
Methodology: We tested several methods for pre-treating urine samples prior to testing the diagnostic performance of the urine OV-ES assay. Using trichloroacetic acid (TCA) pre-treated urine, we compared detection and quantification of OV infection using the urine OV-ES assay versus FECT in OV-endemic areas in Northeastern Thailand. Receiver operating characteristic (ROC) curves were used to determine the diagnostic sensitivity and specificity of the urine OV-ES assay using TCA pre-treated urine, and to establish diagnostic positivity thresholds. The Positive Predictive Value as well as the likelihood of obtaining a positive test result (LR+) or a negative test result (LR-) were calculated for the established diagnostic positivity threshold. Diagnostic risks (Odds Ratios) were estimated using logistic regression.
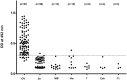
Results: When urine samples were pre-treated with TCA prior to use in the urine OV-ES assay, the analytical sensitivity was significantly improved. Using TCA pre-treatment of urine, the urine OV-ES assay had a limit of detection (LoD) of 39 ng/ml compared to the LoD of 52 ng/mL reported for coprological antigen detection methods. Similarly, the urine OV-ES assay correlated significantly with intensity of OV infection as measured by FECT. The urine OV-ES assay was also able to detect 28 individuals as positive from the 63 (44.4%) individuals previously determined to be negative using FECT. The likelihood of a positive diagnosis of OV infection by urine OV-ES assay increased significantly with the intensity of OV infection as determined by FECT. With reference to FECT, the sensitivity and specificity of the urine OV-ES assay was 81% and 70%, respectively.
Conclusion: The detection of OV-infection by the urine OV-ES assay showed much greater diagnostic sensitivity and diagnostic specificity than the current "gold standard" FECT method for the detection and quantification of OV infection. Due to its ease-of-use, and noninvasive sample collection (urine), the urine OV-ES assay offers the potential to revolutionize the diagnosis of liver fluke infection and provide an effective tool for control and elimination of these tumorigenic parasites.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Large-scale epidemiology of opisthorchiasis in 21 provinces in Thailand based on diagnosis by fecal egg examination and urine antigen assay and analysis of risk factors for infection.PLoS Negl Trop Dis. 2025 Jul 16;19(7):e0013095. doi: 10.1371/journal.pntd.0013095. eCollection 2025 Jul. PLoS Negl Trop Dis. 2025. PMID: 40668872 Free PMC article.
-
Improved performance and quantitative detection of copro-antigens by a monoclonal antibody based ELISA to diagnose human opisthorchiasis.Acta Trop. 2013 Dec;128(3):659-65. doi: 10.1016/j.actatropica.2013.09.012. Epub 2013 Sep 18. Acta Trop. 2013. PMID: 24055716
-
Accuracy of a new rapid diagnostic test for urinary antigen detection and assessment of drug treatment in opisthorchiasis.Infect Dis Poverty. 2023 Nov 21;12(1):102. doi: 10.1186/s40249-023-01162-4. Infect Dis Poverty. 2023. PMID: 37990282 Free PMC article.
-
Immunology and molecular biology of Opisthorchis viverrini infection.Acta Trop. 2003 Nov;88(3):195-207. doi: 10.1016/j.actatropica.2003.02.002. Acta Trop. 2003. PMID: 14611874 Review.
-
Current status of human liver fluke infections in the Greater Mekong Subregion.Acta Trop. 2021 Dec;224:106133. doi: 10.1016/j.actatropica.2021.106133. Epub 2021 Sep 10. Acta Trop. 2021. PMID: 34509453 Review.
Cited by
-
Epidemiology of Strongyloides stercoralis and Opisthorchis viverrini infections in northern and northeastern Thailand: Insights from urine-ELISA surveys.Parasitol Res. 2024 Dec 23;123(12):417. doi: 10.1007/s00436-024-08427-3. Parasitol Res. 2024. PMID: 39714601
-
Clonorchiasis and opisthorchiasis: epidemiology, transmission, clinical features, morbidity, diagnosis, treatment, and control.Clin Microbiol Rev. 2024 Mar 14;37(1):e0000923. doi: 10.1128/cmr.00009-23. Epub 2024 Jan 3. Clin Microbiol Rev. 2024. PMID: 38169283 Free PMC article. Review.
-
Long-term Persistence of Opisthorchis viverrini Antigen in Urine: A Prospective Study in Northeast Thailand.Am J Trop Med Hyg. 2022 Dec 26;108(2):356-358. doi: 10.4269/ajtmh.22-0478. Print 2023 Feb 1. Am J Trop Med Hyg. 2022. PMID: 36572008 Free PMC article.
-
Surveillance and diagnosis of zoonotic foodborne parasites.Food Sci Nutr. 2017 Nov 12;6(1):3-17. doi: 10.1002/fsn3.530. eCollection 2018 Jan. Food Sci Nutr. 2017. PMID: 29387356 Free PMC article. Review.
-
Improvement of a PCR-based method for the detection of Opisthorchis viverrini eggs in human stool samples by targeting internal transcribed spacer-2 (ITS-2), cytochrome oxidase subunit 1 (cox1), and cytochrome b (cyb).J Parasit Dis. 2021 Jun;45(2):474-478. doi: 10.1007/s12639-020-01329-y. Epub 2021 Jan 2. J Parasit Dis. 2021. PMID: 34295047 Free PMC article.
References
-
- Jongsuksuntigul P, Imsomboon T. Opisthorchiasis control in Thailand. Acta Trop. 2003;88(3):229–232. - PubMed
-
- Elkins DB, Haswell-Elkins MR, Mairiang E, Mairiang P, Sithithaworn P, Kaewkes S, et al. A high frequency of hepatobiliary disease and suspected cholangiocarcinoma associated with heavy Opisthorchis viverrini infection in a small community in Northeast, Thailand. Trans R Soc Trop Med Hyg. 1990;84(5):715–719. - PubMed
-
- Sithithaworn P, Haswell-Elkins M. Epidemiology of Opisthorchis viverrini . Acta Trop. 2003;88(3):187–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

