Ibuprofen and Diclofenac Restrict Migration and Proliferation of Human Glioma Cells by Distinct Molecular Mechanisms
- PMID: 26485029
- PMCID: PMC4617646
- DOI: 10.1371/journal.pone.0140613
Ibuprofen and Diclofenac Restrict Migration and Proliferation of Human Glioma Cells by Distinct Molecular Mechanisms
Abstract
Background: Non-steroidal anti-inflammatory drugs (NSAIDs) have been associated with anti-tumorigenic effects in different tumor entities. For glioma, research has generally focused on diclofenac; however data on other NSAIDs, such as ibuprofen, is limited. Therefore, we performed a comprehensive investigation of the cellular, molecular, and metabolic effects of ibuprofen and diclofenac on human glioblastoma cells.
Methods: Glioma cell lines were treated with ibuprofen or diclofenac to investigate functional effects on proliferation and cell motility. Cell cycle, extracellular lactate levels, lactate dehydrogenase-A (LDH-A) expression and activity, as well as inhibition of the Signal Transducer and Activator of Transcription 3 (STAT-3) signaling pathway, were determined. Specific effects of diclofenac and ibuprofen on STAT-3 were investigated by comparing their effects with those of the specific STAT-3 inhibitor STATTIC.
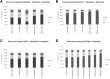
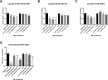
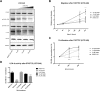
Results: Ibuprofen treatment led to a stronger inhibition of cell growth and migration than treatment with diclofenac. Proliferation was affected by cell cycle arrest at different checkpoints by both agents. In addition, diclofenac, but not ibuprofen, decreased lactate levels in all concentrations used. Both decreased STAT-3 phosphorylation; however, diclofenac led to decreased c-myc expression and subsequent reduction in LDH-A activity, whereas treatment with ibuprofen in higher doses induced c-myc expression and less LDH-A alteration.
Conclusions: This study indicates that both ibuprofen and diclofenac strongly inhibit glioma cells, but the subsequent metabolic responses of both agents are distinct. We postulate that ibuprofen may inhibit tumor cells also by COX- and lactate-independent mechanisms after long-term treatment in physiological dosages, whereas diclofenac mainly acts by inhibition of STAT-3 signaling and downstream modulation of glycolysis.
Conflict of interest statement
Figures




References
-
- Harris RE, Namboodiri KK, Farrar WB. Nonsteroidal antiinflammatory drugs and breast cancer. Epidemiology. 1996;7: 203–205. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

