Activation and lysis of human CD4 cells latently infected with HIV-1
- PMID: 26485194
- PMCID: PMC4633990
- DOI: 10.1038/ncomms9447
Activation and lysis of human CD4 cells latently infected with HIV-1
Abstract
The treatment of AIDS with combination antiretroviral therapy (cART) remains lifelong largely because the virus persists in latent reservoirs. Elimination of latently infected cells could therefore reduce treatment duration and facilitate immune reconstitution. Here we report an approach to reduce the viral reservoir by activating dormant viral gene expression and directing T lymphocytes to lyse previously latent, HIV-1-infected cells. An immunomodulatory protein was created that combines the specificity of a HIV-1 broadly neutralizing antibody with that of an antibody to the CD3 component of the T-cell receptor. CD3 engagement by the protein can stimulate T-cell activation that induces proviral gene expression in latently infected T cells. It further stimulates CD8 T-cell effector function and redirects T cells to lyse these previously latent-infected cells through recognition of newly expressed Env. This immunomodulatory protein could potentially help to eliminate latently infected cells and deplete the viral reservoir in HIV-1-infected individuals.
Figures

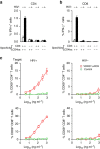
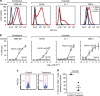
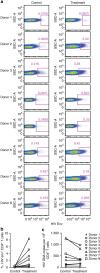
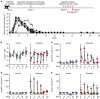
References
-
- Chun T. W. & Fauci A. S. HIV reservoirs: pathogenesis and obstacles to viral eradication and cure. AIDS 26, 1261–1268 (2012). - PubMed
-
- van Praag R. M. et al. OKT3 and IL-2 treatment for purging of the latent HIV-1 reservoir in vivo results in selective long-lasting CD4+ T cell depletion. J. Clin. Immunol. 21, 218–226 (2001). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

