Results From the NACC Uniform Data Set Neuropsychological Battery Crosswalk Study
- PMID: 26485498
- PMCID: PMC4834278
- DOI: 10.1097/WAD.0000000000000111
Results From the NACC Uniform Data Set Neuropsychological Battery Crosswalk Study
Abstract
Introduction: Four new nonproprietary tests were recommended for use in the National Alzheimer's Coordinating Center's Uniform Data Set Neuropsychological Battery. These tests are similar to previous tests but also allow for continuity of longitudinal data collection and wide dissemination among research collaborators.
Methods: A Crosswalk Study was conducted in early 2014 to assess the correlation between each set of new and previous tests. Tests with good correlation were equated using equipercentile equating. The resulting conversion tables allow scores on the new tests to be converted to equivalent scores on the previous tests.
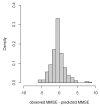
Results: All pairs of tests had good correlation (ρ=0.68 to 0.78). Learning effects were detected for Logical Memory only. Confidence intervals were narrow at each point estimate, and prediction accuracy was high.
Discussion: The recommended new tests are well correlated with the previous tests. The equipercentile equating method produced conversion tables that provide a useful reference for clinicians and researchers.
Figures

References
-
- Beekly DL, Ramos EM, Lee WW, et al. The National Alzheimer’s Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord. 2007;21(3):249–258. - PubMed
-
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. - PubMed
-
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. - PubMed
-
- Wechsler A. Wechsler Adult Intelligence Scale-Revised. San Antonio, TX: Psychological Corporation; 1987.
Publication types
MeSH terms
Grants and funding
- P30 AG013854/AG/NIA NIH HHS/United States
- P30 AG053760/AG/NIA NIH HHS/United States
- P30 AG010124/AG/NIA NIH HHS/United States
- P50 AG023501/AG/NIA NIH HHS/United States
- P50 AG005142/AG/NIA NIH HHS/United States
- P50 AG005131/AG/NIA NIH HHS/United States
- P30 AG010133/AG/NIA NIH HHS/United States
- P50 AG016574/AG/NIA NIH HHS/United States
- P50 AG005146/AG/NIA NIH HHS/United States
- P30 AG035982/AG/NIA NIH HHS/United States
- P50 AG008702/AG/NIA NIH HHS/United States
- U01 AG016976/AG/NIA NIH HHS/United States
- P30 AG008051/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- P30 AG013846/AG/NIA NIH HHS/United States
- P50 AG005136/AG/NIA NIH HHS/United States
- P30 AG012300/AG/NIA NIH HHS/United States
- P50 AG016573/AG/NIA NIH HHS/United States
- P50 AG016570/AG/NIA NIH HHS/United States
- P50 AG005134/AG/NIA NIH HHS/United States
- P30 AG008017/AG/NIA NIH HHS/United States
- P30 AG010161/AG/NIA NIH HHS/United States
- P50 AG025688/AG/NIA NIH HHS/United States
- P50 AG005133/AG/NIA NIH HHS/United States
- P50 AG005138/AG/NIA NIH HHS/United States
- P30 AG010129/AG/NIA NIH HHS/United States
- P30 AG019610/AG/NIA NIH HHS/United States
- P30 AG028383/AG/NIA NIH HHS/United States
- P50 AG033514/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

