Mice with Hepatic Loss of the Desmosomal Protein γ-Catenin Are Prone to Cholestatic Injury and Chemical Carcinogenesis
- PMID: 26485505
- PMCID: PMC4729235
- DOI: 10.1016/j.ajpath.2015.08.019
Mice with Hepatic Loss of the Desmosomal Protein γ-Catenin Are Prone to Cholestatic Injury and Chemical Carcinogenesis
Abstract
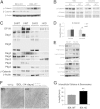
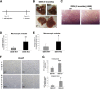
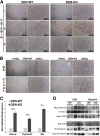
γ-Catenin, an important component of desmosomes, may also participate in Wnt signaling. Herein, we dissect the role of γ-catenin in liver by generating conditional γ-catenin knockout (KO) mice and assessing their phenotype after bile duct ligation (BDL) and diethylnitrosamine-induced chemical carcinogenesis. At baseline, KO and wild-type littermates showed comparable serum biochemistry, liver histology, and global gene expression. β-Catenin protein was modestly increased without any change in Wnt signaling. Desmosomes were maintained in KO, and despite no noticeable changes in gene expression, differential detergent fractionation revealed quantitative and qualitative changes in desmosomal cadherins, plaque proteins, and β-catenin. Enhanced association of β-catenin to desmoglein-2 and plakophilin-3 was observed in KO. When subjected to BDL, wild-type littermates showed specific changes in desmosomal protein expression. In KO, BDL deteriorated baseline compensatory changes, which manifested as enhanced injury and fibrosis. KO also showed enhanced tumorigenesis to diethylnitrosamine treatment because of Wnt activation, as also verified in vitro. γ-Catenin overexpression in hepatoma cells increased its binding to T-cell factor 4 at the expense of β-catenin-T-cell factor 4 association, induced unique target genes, affected Wnt targets, and reduced cell proliferation and viability. Thus, γ-catenin loss in liver is basally well tolerated. However, after insults like BDL, these compensations at desmosomes fail, and KO show enhanced injury. Also, γ-catenin negatively regulates tumor growth by affecting Wnt signaling.
Copyright © 2015 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Bierkamp C., Schwarz H., Huber O., Kemler R. Desmosomal localization of beta-catenin in the skin of plakoglobin null-mutant mice. Development. 1999;126:371–381. - PubMed
-
- Tan X., Behari J., Cieply B., Michalopoulos G.K., Monga S.P. Conditional deletion of beta-catenin reveals its role in liver growth and regeneration. Gastroenterology. 2006;131:1561–1572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

