Anti CD163+, Iba1+, and CD68+ Cells in the Adult Human Inner Ear: Normal Distribution of an Unappreciated Class of Macrophages/Microglia and Implications for Inflammatory Otopathology in Humans
- PMID: 26485593
- PMCID: PMC4675683
- DOI: 10.1097/MAO.0000000000000879
Anti CD163+, Iba1+, and CD68+ Cells in the Adult Human Inner Ear: Normal Distribution of an Unappreciated Class of Macrophages/Microglia and Implications for Inflammatory Otopathology in Humans
Abstract
Hypothesis: Identification, characterization, and location of cells involved in the innate immune defense system of the human inner ear may lead to a better understanding of many otologic diseases and new treatments for hearing and balance-related disorders.
Background: Many otologic disorders are thought to have, as part of their disease process, an immune component. Although resident macrophages are known to exist in the mouse inner ear, the innate immune cells in the human inner ear are, to date, unknown.
Methods: Primary antibodies against CD163, Iba1, and CD68 (markers known to be specific for macrophages/microglia) were used to immunohistochemically stain celloidin embedded archival temporal bone tissue of normal individuals with no known otologic disorders other than changes associated with age.
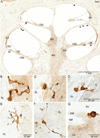
Results: Cells were positively stained throughout the temporal bone within the connective tissue and supporting cells with all three markers. They were often associated with neurons and on occasion entered the sensory cell areas of the auditory and vestibular epithelium.
Conclusions: We have immunohistochemically identified an unappreciated class of cells in the normal adult inner ear consistent in staining characteristics and morphology with macrophages/microglia. As in other organ systems, it is likely these cells play an essential role in organ homeostasis that has not yet been elucidated within the ear.
Conflict of interest statement
There are no declared conflicts of interest.
Figures



Similar articles
-
The Human Endolymphatic Sac and Inner Ear Immunity: Macrophage Interaction and Molecular Expression.Front Immunol. 2019 Feb 1;9:3181. doi: 10.3389/fimmu.2018.03181. eCollection 2018. Front Immunol. 2019. PMID: 30774637 Free PMC article.
-
Phenotypic profile of alternative activation marker CD163 is different in Alzheimer's and Parkinson's disease.Acta Neuropathol Commun. 2014 Feb 14;2:21. doi: 10.1186/2051-5960-2-21. Acta Neuropathol Commun. 2014. PMID: 24528486 Free PMC article.
-
Microglia and brain macrophages are differentially associated with tumor necrosis in glioblastoma: A link to tumor progression.Oncol Res. 2025 Mar 19;33(4):937-950. doi: 10.32604/or.2024.056436. eCollection 2025. Oncol Res. 2025. PMID: 40191733 Free PMC article.
-
The neuropathological basis to the functional role of microglia/macrophages in gliomas.Neurol Sci. 2017 Sep;38(9):1571-1577. doi: 10.1007/s10072-017-3002-x. Epub 2017 Jun 7. Neurol Sci. 2017. PMID: 28593528 Review.
-
The macrophage scavenger receptor CD163.Immunobiology. 2005;210(2-4):153-60. doi: 10.1016/j.imbio.2005.05.010. Immunobiology. 2005. PMID: 16164022 Review.
Cited by
-
Human Inner Ear Immune Activity: A Super-Resolution Immunohistochemistry Study.Front Neurol. 2019 Jul 10;10:728. doi: 10.3389/fneur.2019.00728. eCollection 2019. Front Neurol. 2019. PMID: 31354608 Free PMC article.
-
Serum/glucocorticoid-inducible kinase 1 deficiency induces NLRP3 inflammasome activation and autoinflammation of macrophages in a murine endolymphatic hydrops model.Nat Commun. 2023 Mar 6;14(1):1249. doi: 10.1038/s41467-023-36949-4. Nat Commun. 2023. PMID: 36872329 Free PMC article.
-
The Human Endolymphatic Sac and Inner Ear Immunity: Macrophage Interaction and Molecular Expression.Front Immunol. 2019 Feb 1;9:3181. doi: 10.3389/fimmu.2018.03181. eCollection 2018. Front Immunol. 2019. PMID: 30774637 Free PMC article.
-
The Price of Immune Responses and the Role of Vitamin D in the Inner Ear.Otol Neurotol. 2019 Jul;40(6):701-709. doi: 10.1097/MAO.0000000000002258. Otol Neurotol. 2019. PMID: 31194714 Free PMC article. Review.
-
Two cell populations participate in clearance of damaged hair cells from the sensory epithelia of the inner ear.Hear Res. 2017 Sep;352:70-81. doi: 10.1016/j.heares.2017.04.006. Epub 2017 Apr 28. Hear Res. 2017. PMID: 28526177 Free PMC article. Review.
References
-
- Lim DJ. Functional morphology of the mucosa of the middle ear and eustachian tube. Ann Otol Rhinol Laryngol. 1976;85(2 Suppl 25 Pt 2):36–43. - PubMed
-
- Masuda M, Yamazaki K, Kanzaki J, Hosoda Y. Immunohistochemical and ultrastructural investigation of the human vestibular dark cell area: roles of subepithelial capillaries and T lymphocyte-melanophage interaction in an immune surveillance system. Anat Rec. 1997;249(2):153–162. - PubMed
-
- Altermatt HJ, Gebbers JO, Muller C, Arnold W, Laissue JA. Human endolymphatic sac: evidence for a role in inner ear immune defence. ORL J Otorhinolaryngol Relat Spec. 1990;52(3):143–148. - PubMed
-
- Rask-Andersen H, Danckwardt-Lillieström N, Friberg U, House W. Lymphocyte-macrophage activity in the human endolymphatic sac. Acta Otolaryngol Suppl. 1991;485:15–17. - PubMed
-
- Jansson B, Rask-Andersen H. Osmotically induced macrophage activity in the endolymphatic sac. On the possible interaction between periaqueductal bone marrow cells and the endolymphatic sac. ORL J Otorhinolaryngol Relat Spec. 1992;54(4):191–197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

