Role of Maternal Antibodies in Infants with Severe Diseases Related to Human Parechovirus Type 3
- PMID: 26485714
- PMCID: PMC4622238
- DOI: 10.3201/eid2111.150267
Role of Maternal Antibodies in Infants with Severe Diseases Related to Human Parechovirus Type 3
Abstract
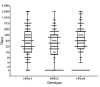
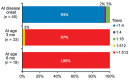
Human parechovirus type 3 (HPeV3) is an emerging pathogen that causes sepsis and meningoencephalitis in young infants. To test the hypothesis that maternal antibodies can protect this population, we measured neutralizing antibody titers (NATs) to HPeV3 and other genotypes (HPeV1 and HPeV6) in 175 cord blood samples in Japan. The seropositivity rate (≥1:32) for HPeV3 was 61%, similar to that for the other genotypes, but decreased significantly as maternal age increased (p<0.001). Furthermore, during the 2014 HPeV3 epidemic, prospective measurement of NATs to HPeV3 in 45 patients with severe diseases caused by HPeV3 infection showed low NATs (≤1:16) at onset and persistently high NATs (≥1:512) until age 6 months. All intravenous immunoglobulin samples tested elicited high NATs to HPeV3. Our findings indicate that maternal antibodies to HPeV3 may help protect young infants from severe diseases related to HPeV3 and that antibody supplementation may benefit these patients.
Keywords: Human parechovirus type 3; antibody; emerging infection; infants; intravenous immunoglobulin; maternal age; meningoencephalitis; neonates; sepsis; viruses.
Figures
Similar articles
-
Human Parechovirus 1, 3 and 4 Neutralizing Antibodies in Dutch Mothers and Infants and Their Role in Protection Against Disease.Pediatr Infect Dis J. 2018 Dec;37(12):1304-1308. doi: 10.1097/INF.0000000000001986. Pediatr Infect Dis J. 2018. PMID: 30382954 Free PMC article.
-
[Human Parechoviruses].Uirusu. 2015;65(1):17-26. doi: 10.2222/jsv.65.17. Uirusu. 2015. PMID: 26923954 Review. Japanese.
-
[Human parechovirus associated sepsis and central nervous system infections in hospitalized children].Zhonghua Er Ke Za Zhi. 2014 Jun;52(6):444-8. Zhonghua Er Ke Za Zhi. 2014. PMID: 25190165 Chinese.
-
Seroepidemiology of human parechovirus types 1, 3, and 6 in Yamagata, Japan, in 2014.Microbiol Immunol. 2016 Dec;60(12):854-858. doi: 10.1111/1348-0421.12456. Microbiol Immunol. 2016. PMID: 27925289
-
Human parechovirus type 3 infection: An emerging infection in neonates and young infants.J Infect Chemother. 2017 Jul;23(7):419-426. doi: 10.1016/j.jiac.2017.04.009. Epub 2017 May 13. J Infect Chemother. 2017. PMID: 28511987 Review.
Cited by
-
An outbreak of severe infections among Australian infants caused by a novel recombinant strain of human parechovirus type 3.Sci Rep. 2017 Mar 14;7:44423. doi: 10.1038/srep44423. Sci Rep. 2017. PMID: 28290509 Free PMC article.
-
Human Parechovirus Associated Critical Illness in Neonates and Infants: Case Series and Review of Literature.Kans J Med. 2023 Nov 30;16(3):289-293. doi: 10.17161/kjm.vol16.21190. eCollection 2023. Kans J Med. 2023. PMID: 38076609 Free PMC article. No abstract available.
-
Human Parechovirus 1, 3 and 4 Neutralizing Antibodies in Dutch Mothers and Infants and Their Role in Protection Against Disease.Pediatr Infect Dis J. 2018 Dec;37(12):1304-1308. doi: 10.1097/INF.0000000000001986. Pediatr Infect Dis J. 2018. PMID: 30382954 Free PMC article.
-
Novel Human Parechovirus 3 Diversity, Recombination, and Clinical Impact Across 7 Years: An Australian Story.J Infect Dis. 2023 Jan 11;227(2):278-287. doi: 10.1093/infdis/jiac311. J Infect Dis. 2023. PMID: 35867852 Free PMC article.
-
Development of Novel PCR Assays for Improved Detection of Enterovirus D68.J Clin Microbiol. 2021 Oct 19;59(11):e0115121. doi: 10.1128/JCM.01151-21. Epub 2021 Aug 25. J Clin Microbiol. 2021. PMID: 34432489 Free PMC article.
References
-
- Racaniello V R. Picornaviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields virology. 6th ed. Vol. 1. Philadelphia: Lippincott, Williams and Wilkins; 2013. p. 610–64.
-
- Japan National Institute of Infectious Diseases. Monthly reports of parechovirus isolation/detection, January 2005. –May 2015 [cited 2015 Apr 13]. http://www.nih.go.jp/niid/images/iasr/rapid/topics/parecho/150611/parech...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

