Catalytic Asymmetric Reactions of 4-Substituted Indoles with Nitroethene: A Direct Entry to Ergot Alkaloid Structures
- PMID: 26486074
- PMCID: PMC4832839
- DOI: 10.1002/chem.201502655
Catalytic Asymmetric Reactions of 4-Substituted Indoles with Nitroethene: A Direct Entry to Ergot Alkaloid Structures
Abstract
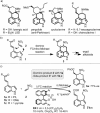
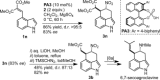
A domino Friedel-Crafts/nitro-Michael reaction between 4-substituted indoles and nitroethene is presented. The reaction is catalyzed by BINOL-derived phosphoric acid catalysts, and delivers the corresponding 3,4-ring-fused indoles with very good results in terms of yields and diastereo- and enantioselectivities. The tricyclic benzo[cd]indole products bear a nitro group at the right position to serve as precursors of ergot alkaloids, as demonstrated by the formal synthesis of 6,7-secoagroclavine from one of the adducts. DFT calculations suggest that the outcome of the reaction stems from the preferential evolution of a key nitronic acid intermediate through a nucleophilic addition pathway, rather than to the expected "quenching" through protonation.
Keywords: Brønsted acids; asymmetric synthesis; indoles; nitroalkenes; organocatalysis.
© 2015 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA. This is an open access article under the terms of Creative Commons Attribution NonCommercial-NoDerivs. License, which permits use and distribution in any medium, provided the original work is properly cited, the use is non-commercial and no modifications or adaptations are made.
Figures


References
-
- Schardl C. L., Panaccione D. G., Tudzynski P. in The Alkaloids, Vol. 63 (Ed.: G. A. Cordell), Academic Press, San Diego, 2006, pp. 45–86. - PubMed
-
- None
-
- Floss H. G., Tetrahedron 1976, 32, 873;
-
- Schwarzer D. D., Gritsch P. J., Gaich T., Synlett 2013, 24, 1025;
-
- Wallwey C., Li S.‐M., Nat. Prod. Rep. 2011, 28, 496. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

