Molecular mechanisms of cisplatin cytotoxicity in acute promyelocytic leukemia cells
- PMID: 26486083
- PMCID: PMC4747365
- DOI: 10.18632/oncotarget.5754
Molecular mechanisms of cisplatin cytotoxicity in acute promyelocytic leukemia cells
Abstract
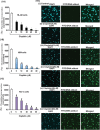
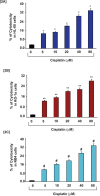
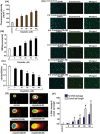
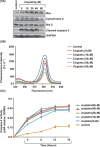
Cis-diamminedichloroplatinum (II) (cisplatin) is a widely used anti-tumor drug for the treatment of a broad range of human malignancies with successful therapeutic outcomes for head and neck, ovarian, and testicular cancers. It has been found to inhibit cell cycle progression and to induce oxidative stress and apoptosis in acute promyelocytic leukemia (APL) cells. However, its molecular mechanisms of cytotoxic action are poorly understood. We hypothesized that cisplatin induces cytotoxicity through DNA adduct formation, oxidative stress, transcriptional factors (p53 and AP-1), cell cycle regulation, stress signaling and apoptosis in APL cells. We used the APL cell line as a model, and applied a variety of molecular tools to elucidate the cytotoxic mode of action of cisplatin. We found that cisplatin inhibited cell proliferation by a cytotoxicity, characterized by DNA damage and modulation of oxidative stress. Cisplatin also activated p53 and phosphorylated activator protein (AP-1) component, c-Jun at serine (63, 73) residue simultaneously leading to cell cycle arrest through stimulation of p21 and down regulation of cyclins and cyclin dependent kinases in APL cell lines. It strongly activated the intrinsic pathway of apoptosis through alteration of the mitochondrial membrane potential, release of cytochrome C, and up-regulation of caspase 3 activity. It also down regulated the p38MAPK pathway. Overall, this study highlights the molecular mechanisms that underline cisplatin toxicity to APL cells, and provides insights into selection of novel targets and/or design of therapeutic agents to treat APL.
Keywords: AP-1 and p53; APL cell line; cell cycle modulation; cisplatin.
Conflict of interest statement
Authors declare no conflict of interest.
Figures

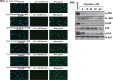
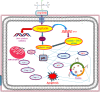
References
-
- Powell BL. Arsenic trioxide in acute promyelocytic leukemia: potion not poison. Expert Rev Anticancer Ther. 2011;11:1317–1319. - PubMed
-
- Ghavamzadeh A, Alimoghaddam K, Ghaffari SH, Rostami S, Jahani M, Hosseini R, Mossavi A, Baybordi E, Khodabadeh A, Iravani M, Bahar B, Mortazavi Y, Totonchi M, Aghdami N. Treatment of acute promyelocytic leukemia with arsenic trioxide without ATRA and/or chemotherapy. Ann Oncol. 2006;17:131–134. - PubMed
-
- Ghavamzadeh A, Alimoghaddam K, Rostami S, Ghaffari SH, Jahani M, Iravani M, Mousavi SA, Bahar B, Jalili M. Phase II study of single-agent arsenic trioxide for the front-line therapy of acute promyelocytic leukemia. J Clin Oncol. 2011;29:2753–2757. - PubMed
-
- Tomita A, Kiyoi H, Naoe T. Mechanisms of action and resistance to all-trans retinoic acid (ATRA) and arsenic trioxide (As2O3) in acute promyelocytic leukemia. Int. J. Hematol. 2013;97:717–725. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

