Ablation of CD8α(+) dendritic cell mediated cross-presentation does not impact atherosclerosis in hyperlipidemic mice
- PMID: 26486587
- PMCID: PMC4614009
- DOI: 10.1038/srep15414
Ablation of CD8α(+) dendritic cell mediated cross-presentation does not impact atherosclerosis in hyperlipidemic mice
Erratum in
-
Author Correction: Ablation of CD8α+ dendritic cell mediated cross-presentation does not impact atherosclerosis in hyperlipidemic mice.Sci Rep. 2020 May 26;10(1):8747. doi: 10.1038/s41598-020-65653-2. Sci Rep. 2020. PMID: 32457341 Free PMC article.
Abstract
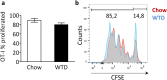
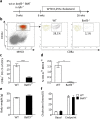
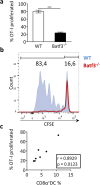
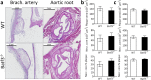
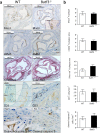
Clinical complications of atherosclerosis are almost exclusively linked to destabilization of the atherosclerotic plaque. Batf3-dependent dendritic cells specialize in cross-presentation of necrotic tissue-derived epitopes to directly activate cytolytic CD8 Tcells. The mature plaque (necrotic, containing dendritic cells and CD8 Tcells) could offer the ideal environment for cross-presentation, resulting in cytotoxic immunity and plaque destabilization. Ldlr(-/-) mice were transplanted with batf3(-/-) or wt bone marrow and put on a western type diet. Hematopoietic batf3 deficiency sharply decreased CD8α(+) DC numbers in spleen and lymph nodes (>80%; P < 0,001). Concordantly, batf3(-/-) chimeras had a 75% reduction in OT-I cross-priming capacity in vivo. Batf3(-/-) chimeric mice did not show lower Tcell or other leukocyte subset numbers. Despite dampened cross-presentation capacity, batf3(-/-) chimeras had equal atherosclerosis burden in aortic arch and root. Likewise, batf3(-/-) chimeras and wt mice revealed no differences in parameters of plaque stability: plaque Tcell infiltration, cell death, collagen composition, and macrophage and vascular smooth muscle cell content were unchanged. These results show that CD8α(+) DC loss in hyperlipidemic mice profoundly reduces cross-priming ability, nevertheless it does not influence lesion development. Taken together, we clearly demonstrate that CD8α(+) DC-mediated cross-presentation does not significantly contribute to atherosclerotic plaque formation and stability.
Figures

Similar articles
-
Deletion of Batf3-dependent antigen-presenting cells does not affect atherosclerotic lesion formation in mice.PLoS One. 2017 Aug 3;12(8):e0181947. doi: 10.1371/journal.pone.0181947. eCollection 2017. PLoS One. 2017. PMID: 28771609 Free PMC article.
-
Batf3-dependent CD8α+ Dendritic Cells Aggravates Atherosclerosis via Th1 Cell Induction and Enhanced CCL5 Expression in Plaque Macrophages.EBioMedicine. 2017 Apr;18:188-198. doi: 10.1016/j.ebiom.2017.04.008. Epub 2017 Apr 6. EBioMedicine. 2017. PMID: 28411140 Free PMC article.
-
Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity.Science. 2008 Nov 14;322(5904):1097-100. doi: 10.1126/science.1164206. Science. 2008. PMID: 19008445 Free PMC article.
-
Concise review: The heterogenous roles of BATF3 in cancer oncogenesis and dendritic cells and T cells differentiation and function considering the importance of BATF3-dependent dendritic cells.Immunogenetics. 2024 Apr;76(2):75-91. doi: 10.1007/s00251-024-01335-x. Epub 2024 Feb 15. Immunogenetics. 2024. PMID: 38358555 Review.
-
CD8+ T Cells in Atherosclerosis.Cells. 2020 Dec 29;10(1):37. doi: 10.3390/cells10010037. Cells. 2020. PMID: 33383733 Free PMC article. Review.
Cited by
-
Functional crosstalk between T cells and monocytes in cancer and atherosclerosis.J Leukoc Biol. 2020 Jul;108(1):297-308. doi: 10.1002/JLB.1MIR0420-076R. Epub 2020 Jun 12. J Leukoc Biol. 2020. PMID: 32531833 Free PMC article. Review.
-
Immunometabolism and atherosclerosis: perspectives and clinical significance: a position paper from the Working Group on Atherosclerosis and Vascular Biology of the European Society of Cardiology.Cardiovasc Res. 2019 Jul 1;115(9):1385-1392. doi: 10.1093/cvr/cvz166. Cardiovasc Res. 2019. PMID: 31228191 Free PMC article. Review.
-
Induction of HLA-A2 restricted CD8 T cell responses against ApoB100 peptides does not affect atherosclerosis in a humanized mouse model.Sci Rep. 2019 Nov 22;9(1):17391. doi: 10.1038/s41598-019-53642-z. Sci Rep. 2019. PMID: 31757993 Free PMC article.
-
CD8+ T Cells Protect During Vein Graft Disease Development.Front Cardiovasc Med. 2019 Jun 13;6:77. doi: 10.3389/fcvm.2019.00077. eCollection 2019. Front Cardiovasc Med. 2019. PMID: 31263704 Free PMC article.
-
Targeting non-coding RNAs in unstable atherosclerotic plaques: Mechanism, regulation, possibilities, and limitations.Int J Biol Sci. 2021 Aug 3;17(13):3413-3427. doi: 10.7150/ijbs.62506. eCollection 2021. Int J Biol Sci. 2021. PMID: 34512156 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

