2-Methoxyestradiol blocks the RhoA/ROCK1 pathway in human aortic smooth muscle cells
- PMID: 26487003
- PMCID: PMC4816197
- DOI: 10.1152/ajpendo.00267.2015
2-Methoxyestradiol blocks the RhoA/ROCK1 pathway in human aortic smooth muscle cells
Abstract
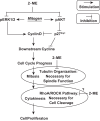
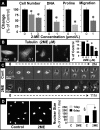
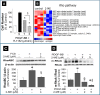
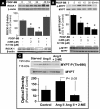
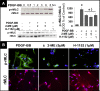
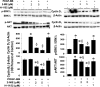
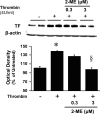
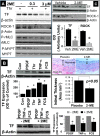
2-Methoxyestradiol (2-ME), a metabolite of estradiol with little affinity for estrogen receptors, inhibits proliferation of vascular smooth muscle cells; however, the molecular mechanisms underlying this effect are incompletely understood. Our previous work shows that 2-ME inhibits initiation (blocks phosphorylation of ERK and Akt) and progression (reduces cyclin expression and increases expression of cyclin inhibitors) of the mitogenic pathway and interferes with mitosis (disrupts tubulin organization). Because the RhoA/ROCK1 pathway (RhoA → ROCK1 → myosin phosphatase targeting subunit → myosin light chain) is involved in cytokinesis, herein we tested the concept that 2-ME also blocks the RhoA/ROCK1 pathway. Because of the potential importance of 2-ME for preventing/treating vascular diseases, experiments were conducted in female human aortic vascular smooth muscle cells. Microarray transcriptional profiling suggested an effect of 2-ME on the RhoA/ROCK1 pathway. Indeed, 2-ME blocked mitogen-induced GTP-bound RhoABC expression and membrane-bound RhoA, suggesting interference with the activation of RhoA. 2-ME also reduced ROCK1 expression, suggesting reduced production of the primary downstream signaling kinase of the RhoA pathway. Moreover, 2-ME inhibited RhoA/ROCK1 pathway downstream signaling, including phosphorylated myosin phosphatase targeting subunit and myosin light chain; the ROCK1 inhibitor H-1152 mimicked these effects of 2-ME; both 2-ME and H-1152 blocked cytokinesis. 2-ME also reduced the expression of tissue factor, yet another downstream signaling component of the RhoA/ROCK1 pathway. We conclude that 2-ME inhibits the pathway RhoA → ROCK1 → myosin phosphatase targeting subunit → myosin light chain, and this likely contributes to the reduced cytokinesis in 2-ME treated HASMCs.
Keywords: 2-methoxyestradiol; ROCK1; RhoA; cytokinesis; myosin light chain; myosin phosphatase targeting subunit.
Copyright © 2015 the American Physiological Society.
Figures

Similar articles
-
2-methoxyestradiol inhibits atorvastatin-induced rounding of human vascular smooth muscle cells.J Cell Physiol. 2010 Mar;222(3):556-64. doi: 10.1002/jcp.21970. J Cell Physiol. 2010. PMID: 19937728
-
Up-regulation of Rhoa/Rho kinase pathway by translationally controlled tumor protein in vascular smooth muscle cells.Int J Mol Sci. 2014 Jun 10;15(6):10365-76. doi: 10.3390/ijms150610365. Int J Mol Sci. 2014. PMID: 24918292 Free PMC article.
-
Resveratrol prevents AngII-induced hypertension via AMPK activation and RhoA/ROCK suppression in mice.Hypertens Res. 2014 Sep;37(9):803-10. doi: 10.1038/hr.2014.90. Epub 2014 Jun 26. Hypertens Res. 2014. PMID: 24965170
-
RhoA/Rho-Kinase in the Cardiovascular System.Circ Res. 2016 Jan 22;118(2):352-66. doi: 10.1161/CIRCRESAHA.115.306532. Circ Res. 2016. PMID: 26838319 Review.
-
Pathophysiological effects of RhoA and Rho-associated kinase on cardiovascular system.J Hypertens. 2016 Jan;34(1):3-10. doi: 10.1097/HJH.0000000000000768. J Hypertens. 2016. PMID: 26556565 Review.
Cited by
-
ZYZ-168 alleviates cardiac fibrosis after myocardial infarction through inhibition of ERK1/2-dependent ROCK1 activation.Sci Rep. 2017 Mar 7;7:43242. doi: 10.1038/srep43242. Sci Rep. 2017. PMID: 28266583 Free PMC article.
-
Decoding the enigmatic estrogen paradox in pulmonary hypertension: delving into estrogen metabolites and metabolic enzymes.Cell Mol Biol Lett. 2024 Dec 18;29(1):155. doi: 10.1186/s11658-024-00671-w. Cell Mol Biol Lett. 2024. PMID: 39695964 Free PMC article. Review.
-
Apolipoprotein A1 mimetic peptide ATI-5261 reverses arterial stiffness at late pregnancy and early postpartum in a COMT-/- mouse model of preeclampsia.Clin Hypertens. 2018 Sep 15;24:11. doi: 10.1186/s40885-018-0097-1. eCollection 2018. Clin Hypertens. 2018. PMID: 30237900 Free PMC article.
-
Transcriptome Profiling in Systems Vascular Medicine.Front Pharmacol. 2017 Aug 25;8:563. doi: 10.3389/fphar.2017.00563. eCollection 2017. Front Pharmacol. 2017. PMID: 28970795 Free PMC article.
-
Nogo receptor knockdown and ciliary neurotrophic factor attenuate diabetic retinopathy in streptozotocin-induced diabetic rats.Mol Med Rep. 2017 Aug;16(2):2030-2036. doi: 10.3892/mmr.2017.6850. Epub 2017 Jun 23. Mol Med Rep. 2017. PMID: 28656312 Free PMC article.
References
-
- Archacki SR, Angheloiu G, Tian XL, Tan FL, DiPaola N, Shen GQ, Moravec C, Ellis S, Topol EJ, Wang Q. Identification of new genes differentially expressed in coronary artery disease by expression profiling. Physiol Genomics 15: 65–74, 2003. - PubMed
-
- Asano S, Hamao K, Hosoya H. Direct evidence for roles of phosphorylated regulatory light chain of myosin II in furrow ingression during cytokinesis in HeLa cells. Genes Cells 14: 555–568, 2009. - PubMed
-
- Attalla H, Makela TP, Adlercreutz H, Andersson LC. 2-Methoxyestradiol arrests cells in mitosis without depolymerizing tubulin. Biochem Biophys Res Commun 228: 467–473, 1996. - PubMed
-
- Barchiesi F, Jackson EK, Fingerle J, Gillespie DG, Odermatt B, Dubey RK. 2-Methoxyestradiol, an estradiol metabolite, inhibits neointima formation and smooth muscle cell growth via double blockade of the cell cycle. Circ Res 99: 266–274, 2006. - PubMed
-
- Barchiesi F, Jackson EK, Gillespie DG, Zacharia LC, Fingerle J, Dubey RK. Methoxyestradiols mediate estradiol-induced antimitogenesis in human aortic SMCs. Hypertension 39: 874–879, 2002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

