Early initiation of chemotherapy following complete resection of advanced ovarian cancer associated with improved survival: NRG Oncology/Gynecologic Oncology Group study
- PMID: 26487588
- PMCID: PMC4684156
- DOI: 10.1093/annonc/mdv500
Early initiation of chemotherapy following complete resection of advanced ovarian cancer associated with improved survival: NRG Oncology/Gynecologic Oncology Group study
Abstract
Background: To determine whether time from surgery to initiation of chemotherapy impacts survival in advanced ovarian carcinoma.
Patients and methods: This is a post-trial ad hoc analysis of Gynecologic Oncology Group protocol 218, a phase III randomized, double-blind, placebo-controlled trial designed to study the antiangiogenesis agent, bevacizumab, in primary and maintenance therapy for patients with newly diagnosed advanced ovarian carcinoma. Maximum attempt at debulking was an eligibility criterion. Stage III patients, not stage IV, were required to have gross macroscopic or palpable residual disease following surgery. The survival impact of time from surgery to initiation of chemotherapy was studied using Cox regression models and stratified by treatment arm, residual disease and other clinical and pathologic factors.
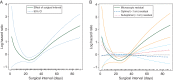
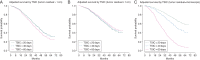
Results: One thousand seven hundred eighteen assessable patients were randomized (stage III (n = 1237); stage IV (n = 477), including those with complete resection (stage IV only, n = 81), low-volume residual (≤1 cm, n = 701), and suboptimal (>1 cm, n = 932). On multivariate analysis, time to chemotherapy initiation was predictive of overall survival (P < 0.001), with the complete resection group (i.e. stage IV) encountering an increased risk of death when time to initiation of chemotherapy exceeded 25 days (95% confidence interval 16.6-49.9 days).
Conclusion: Survival for women with advanced ovarian cancer may be adversely affected when initiation of chemotherapy occurs >25 days following surgery. Our analysis applies to stage IV only as women with stage III who underwent complete resection were not eligible for this trial. These results, however, are consistent with Gompertzian first-order kinetics where patients with microscopic residual are most vulnerable.
Clinical trials identifier: NCT00262847.
Keywords: NRG Oncology/GOG; chemotherapy initiation; complete resection; ovarian cancer.
© The Author 2015. Published by Oxford University Press on behalf of the European Society for Medical Oncology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65: 5–29. - PubMed
-
- Mahner S, Eulenburg C, Staehle A et al. . Prognostic impact of the time interval between surgery and chemotherapy in advanced ovarian cancer: analysis of prospective randomised phase III trials. Eur J Cancer 2013; 49: 142–149. - PubMed
-
- Burger RA, Brady MF, Bookman MA et al. . Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 2011; 365: 2473–2483. - PubMed
-
- Eskander RN, Tewari KS. Incorporation of anti-angiogenesis therapy in the management of advanced ovarian carcinoma: mechanistics, review of phase 3 randomized clinical trials, and regulatory implications. Gynecol Oncol 2014; 132: 496–505. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

