Prediabetes linked to excess glucagon in transgenic mice with pancreatic active AKT1
- PMID: 26487674
- PMCID: PMC4803065
- DOI: 10.1530/JOE-15-0288
Prediabetes linked to excess glucagon in transgenic mice with pancreatic active AKT1
Abstract
Protein kinase B/AKT has three isoforms (AKT1-3) and is renowned for its central role in the regulation of cell growth and proliferation, due to its constitutive activation in various cancers. AKT2, which is highly expressed in insulin-responsive tissues, has been identified as a primary regulator of glucose metabolism as Akt2 knockout mice (Akt2(-/-)) are glucose-intolerant and insulin-resistant. However, the role of AKT1 in glucose metabolism is not as clearly defined. We previously showed that mice with myristoylated Akt1 (AKT1(Myr)) expressed through a bicistronic Pdx1-TetA and TetO-MyrAkt1 system were susceptible to islet cell carcinomas, and in this study we characterized an early onset, prediabetic phenotype. Beginning at weaning (3 weeks of age), the glucose-intolerant AKT1(Myr) mice exhibited non-fasted hyperglycemia, which progressed to fasted hyperglycemia by 5 months of age. The glucose intolerance was attributed to a fasted hyperglucagonemia, and hepatic insulin resistance detectable by reduced phosphorylation of the insulin receptor following insulin injection into the inferior vena cava. In contrast, treatment with doxycycline diet to turn off the transgene caused attenuation of the non-fasted and fasted hyperglycemia, thus affirming AKT1 hyperactivation as the trigger. Collectively, this model highlights a novel glucagon-mediated mechanism by which AKT1 hyperactivation affects glucose homeostasis and provides an avenue to better delineate the molecular mechanisms responsible for diabetes mellitus and the potential association with pancreatic cancer.
Keywords: glucagon; glucose metabolism; insulin signaling; oncogenes; pancreas.
© 2016 Society for Endocrinology.
Conflict of interest statement
There are no conflicts of interest biasing the research reported.
Figures
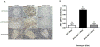

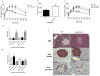


References
-
- Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–7464. - PubMed
-
- Baron AD, Schaeffer L, Shragg P, Kolterman OG. Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics. Diabetes. 1987;36:274–283. - PubMed
-
- Basu A, Basu R, Shah P, Vella A, Johnson CM, Nair KS, Jensen MD, Schwenk WF, Rizza RA. Effects of type 2 diabetes on the ability of insulin and glucose to regulate splanchnic and muscle glucose metabolism: evidence for a defect in hepatic glucokinase activity. Diabetes. 2000;49:272–283. - PubMed
-
- Basu R, Basu A, Johnson CM, Schwenk WF, Rizza RA. Insulin dose-response curves for stimulation of splanchnic glucose uptake and suppression of endogenous glucose production differ in nondiabetic humans and are abnormal in people with type 2 diabetes. Diabetes. 2004;53:2042–2050. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

