Knockout of Lmod2 results in shorter thin filaments followed by dilated cardiomyopathy and juvenile lethality
- PMID: 26487682
- PMCID: PMC4640780
- DOI: 10.1073/pnas.1508273112
Knockout of Lmod2 results in shorter thin filaments followed by dilated cardiomyopathy and juvenile lethality
Abstract
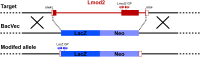
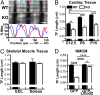
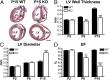
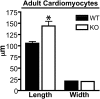
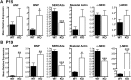
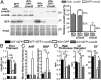
Leiomodin 2 (Lmod2) is an actin-binding protein that has been implicated in the regulation of striated muscle thin filament assembly; its physiological function has yet to be studied. We found that knockout of Lmod2 in mice results in abnormally short thin filaments in the heart. We also discovered that Lmod2 functions to elongate thin filaments by promoting actin assembly and dynamics at thin filament pointed ends. Lmod2-KO mice die as juveniles with hearts displaying contractile dysfunction and ventricular chamber enlargement consistent with dilated cardiomyopathy. Lmod2-null cardiomyocytes produce less contractile force than wild type when plated on micropillar arrays. Introduction of GFP-Lmod2 via adeno-associated viral transduction elongates thin filaments and rescues structural and functional defects observed in Lmod2-KO mice, extending their lifespan to adulthood. Thus, to our knowledge, Lmod2 is the first identified mammalian protein that functions to elongate actin filaments in the heart; it is essential for cardiac thin filaments to reach a mature length and is required for efficient contractile force and proper heart function during development.
Keywords: actin-thin filaments; cardiomyopathy; cytoskeletal dynamics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Gregorio CC, Weber A, Bondad M, Pennise CR, Fowler VM. Requirement of pointed-end capping by tropomodulin to maintain actin filament length in embryonic chick cardiac myocytes. Nature. 1995;377(6544):83–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

