Normal Fertility Requires the Expression of Carbonic Anhydrases II and IV in Sperm
- PMID: 26487715
- PMCID: PMC4705926
- DOI: 10.1074/jbc.M115.698597
Normal Fertility Requires the Expression of Carbonic Anhydrases II and IV in Sperm
Abstract
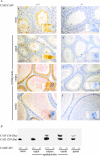
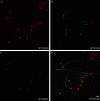
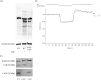
HCO3 (-) is a key factor in the regulation of sperm motility. High concentrations of HCO3 (-) in the female genital tract induce an increase in sperm beat frequency, which speeds progress of the sperm through the female reproductive tract. Carbonic anhydrases (CA), which catalyze the reversible hydration of CO2 to HCO3 (-), represent potential candidates in the regulation of the HCO3 (-) homeostasis in sperm and the composition of the male and female genital tract fluids. We show that two CA isoforms, CAII and CAIV, are distributed along the epididymal epithelium and appear with the onset of puberty. Expression analyses reveal an up-regulation of CAII and CAIV in the different epididymal sections of the knockout lines. In sperm, we find that CAII is located in the principal piece, whereas CAIV is present in the plasma membrane of the entire sperm tail. CAII and CAIV single knockout animals display an imbalanced HCO3 (-) homeostasis, resulting in substantially reduced sperm motility, swimming speed, and HCO3 (-)-enhanced beat frequency. The CA activity remaining in the sperm of CAII- and CAIV-null mutants is 35% and 68% of that found in WT mice. Sperm of the double knockout mutant mice show responses to stimulus by HCO3 (-) or CO2 that were delayed in onset and reduced in magnitude. In comparison with sperm from CAII and CAIV double knockout animals, pharmacological loss of CAIV in sperm from CAII knockout animals, show an even lower response to HCO3 (-). These results suggest that CAII and CAIV are required for optimal fertilization.
Keywords: bicarbonate; carbon dioxide; carbonic anhydrase; fertilization; mutant; sperm; subfertility.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





Similar articles
-
Intracellular and extracellular carbonic anhydrases cooperate non-enzymatically to enhance activity of monocarboxylate transporters.J Biol Chem. 2014 Jan 31;289(5):2765-75. doi: 10.1074/jbc.M113.537043. Epub 2013 Dec 12. J Biol Chem. 2014. PMID: 24338019 Free PMC article.
-
Transport activity of the high-affinity monocarboxylate transporter MCT2 is enhanced by extracellular carbonic anhydrase IV but not by intracellular carbonic anhydrase II.J Biol Chem. 2011 Aug 5;286(31):27781-91. doi: 10.1074/jbc.M111.255331. Epub 2011 Jun 16. J Biol Chem. 2011. PMID: 21680735 Free PMC article.
-
Carbonic Anhydrase IV Deficiency Causes Intrauterine Embryonic Loss in Mice.Cells Tissues Organs. 2025;214(4):287-297. doi: 10.1159/000544000. Epub 2025 Feb 5. Cells Tissues Organs. 2025. PMID: 39908001 Free PMC article.
-
pH Homeodynamics and Male Fertility: A Coordinated Regulation of Acid-Based Balance during Sperm Journey to Fertilization.Biomolecules. 2024 Jun 12;14(6):685. doi: 10.3390/biom14060685. Biomolecules. 2024. PMID: 38927088 Free PMC article. Review.
-
The bicarbonate transport metabolon.J Enzyme Inhib Med Chem. 2004 Jun;19(3):231-6. doi: 10.1080/14756360410001704443. J Enzyme Inhib Med Chem. 2004. PMID: 15499994 Review.
Cited by
-
Cytosolic and Acrosomal pH Regulation in Mammalian Sperm.Cells. 2024 May 17;13(10):865. doi: 10.3390/cells13100865. Cells. 2024. PMID: 38786087 Free PMC article. Review.
-
The Mammalian Spermatogenesis Single-Cell Transcriptome, from Spermatogonial Stem Cells to Spermatids.Cell Rep. 2018 Nov 6;25(6):1650-1667.e8. doi: 10.1016/j.celrep.2018.10.026. Cell Rep. 2018. PMID: 30404016 Free PMC article.
-
Control of intracellular pH and bicarbonate by CO2 diffusion into human sperm.Nat Commun. 2023 Sep 5;14(1):5395. doi: 10.1038/s41467-023-40855-0. Nat Commun. 2023. PMID: 37669933 Free PMC article.
-
CFTR/ENaC-dependent regulation of membrane potential during human sperm capacitation is initiated by bicarbonate uptake through NBC.J Biol Chem. 2018 Jun 22;293(25):9924-9936. doi: 10.1074/jbc.RA118.003166. Epub 2018 May 9. J Biol Chem. 2018. PMID: 29743243 Free PMC article.
-
Insights into pH regulatory mechanisms in mediating spermatozoa functions.Vet World. 2018 Jun;11(6):852-858. doi: 10.14202/vetworld.2018.852-858. Epub 2018 Jun 26. Vet World. 2018. PMID: 30034181 Free PMC article. Review.
References
-
- Sullivan R., and Saez F. (2013) Epididymosomes, prostasomes, and liposomes: their roles in mammalian male reproductive physiology. Reproduction 146, R21–35 - PubMed
-
- Tulsiani D. R., and Abou-Haila A. (2011) Molecular events that regulate mammalian fertilization. Minerva Ginecologica 63, 103–118 - PubMed
-
- Visconti P. E., Bailey J. L., Moore G. D., Pan D., Olds-Clarke P., and Kopf G. S. (1995) Capacitation of mouse spermatozoa: 1: correlation between the capacitation state and protein-tyrosine phosphorylation. Development 121, 1129–1137 - PubMed
-
- Visconti P. E., Moore G. D., Bailey J. L., Leclerc P., Connors S. A., Pan D., Olds-Clarke P., and Kopf G. S. (1995) Capacitation of mouse spermatozoa: 2: protein-tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development 121, 1139–1150 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

