N-helix and Cysteines Inter-regulate Human Mitochondrial VDAC-2 Function and Biochemistry
- PMID: 26487717
- PMCID: PMC4683249
- DOI: 10.1074/jbc.M115.693978
N-helix and Cysteines Inter-regulate Human Mitochondrial VDAC-2 Function and Biochemistry
Abstract
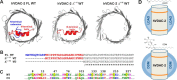
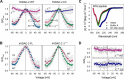
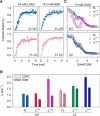
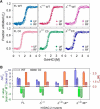
Human voltage-dependent anion channel-2 (hVDAC-2) functions primarily as the crucial anti-apoptotic protein in the outer mitochondrial membrane, and additionally as a gated bidirectional metabolite transporter. The N-terminal helix (NTH), involved in voltage sensing, bears an additional 11-residue extension (NTE) only in hVDAC-2. In this study, we assign a unique role for the NTE as influencing the chaperone-independent refolding kinetics and overall thermodynamic stability of hVDAC-2. Our electrophysiology data shows that the N-helix is crucial for channel activity, whereas NTE sensitizes this isoform to voltage gating. Additionally, hVDAC-2 possesses the highest cysteine content, possibly for regulating reactive oxygen species content. We identify interdependent contributions of the N-helix and cysteines to channel function, and the measured stability in micellar environments with differing physicochemical properties. The evolutionary demand for the NTE in the presence of cysteines clearly emerges from our biochemical and functional studies, providing insight into factors that functionally demarcate hVDAC-2 from the other VDACs.
Keywords: N-terminal domain; gating; ion channel; membrane protein; protein folding; thermodynamics; voltage-dependent anion channel (VDAC).
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



Similar articles
-
Influence of protein-micelle ratios and cysteine residues on the kinetic stability and unfolding rates of human mitochondrial VDAC-2.PLoS One. 2014 Jan 29;9(1):e87701. doi: 10.1371/journal.pone.0087701. eCollection 2014. PLoS One. 2014. PMID: 24494036 Free PMC article.
-
Cysteine residues impact the stability and micelle interaction dynamics of the human mitochondrial β-barrel anion channel hVDAC-2.PLoS One. 2014 Mar 18;9(3):e92183. doi: 10.1371/journal.pone.0092183. eCollection 2014. PLoS One. 2014. PMID: 24642864 Free PMC article.
-
Modulation of human mitochondrial voltage-dependent anion channel 2 (hVDAC-2) structural stability by cysteine-assisted barrel-lipid interactions.J Biol Chem. 2013 Aug 30;288(35):25584-25592. doi: 10.1074/jbc.M113.493692. Epub 2013 Jul 19. J Biol Chem. 2013. PMID: 23873934 Free PMC article.
-
Role of cysteines in mammalian VDAC isoforms' function.Biochim Biophys Acta. 2016 Aug;1857(8):1219-1227. doi: 10.1016/j.bbabio.2016.02.020. Epub 2016 Mar 4. Biochim Biophys Acta. 2016. PMID: 26947058 Free PMC article. Review.
-
VDAC-2: Mitochondrial outer membrane regulator masquerading as a channel?FEBS J. 2016 May;283(10):1831-6. doi: 10.1111/febs.13637. Epub 2016 Jan 13. FEBS J. 2016. PMID: 26709731 Free PMC article. Review.
Cited by
-
Targeting the Multiple Physiologic Roles of VDAC With Steroids and Hydrophobic Drugs.Front Physiol. 2020 May 7;11:446. doi: 10.3389/fphys.2020.00446. eCollection 2020. Front Physiol. 2020. PMID: 32457654 Free PMC article. Review.
-
Reversible folding energetics of Yersinia Ail barrel reveals a hyperfluorescent intermediate.Biochim Biophys Acta Biomembr. 2020 Feb 1;1862(2):183097. doi: 10.1016/j.bbamem.2019.183097. Epub 2019 Oct 28. Biochim Biophys Acta Biomembr. 2020. PMID: 31672545 Free PMC article.
-
Cysteine Oxidations in Mitochondrial Membrane Proteins: The Case of VDAC Isoforms in Mammals.Front Cell Dev Biol. 2020 Jun 4;8:397. doi: 10.3389/fcell.2020.00397. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32582695 Free PMC article. Review.
-
Transmembrane β-barrels: Evolution, folding and energetics.Biochim Biophys Acta Biomembr. 2017 Dec;1859(12):2467-2482. doi: 10.1016/j.bbamem.2017.09.020. Epub 2017 Sep 22. Biochim Biophys Acta Biomembr. 2017. PMID: 28943271 Free PMC article. Review.
-
Conformational plasticity of mitochondrial VDAC2 controls the kinetics of its interaction with cytosolic proteins.Sci Adv. 2025 Apr 25;11(17):eadv4410. doi: 10.1126/sciadv.adv4410. Epub 2025 Apr 23. Sci Adv. 2025. PMID: 40267181 Free PMC article.
References
-
- Messina A., Reina S., Guarino F., and De Pinto V. (2012) VDAC isoforms in mammals. Biochim. Biophys. Acta 1818, 1466–1476 - PubMed
-
- Shoshan-Barmatz V., Ben-Hail D., Admoni L., Krelin Y., and Tripathi S. S. (2015) The mitochondrial voltage-dependent anion channel 1 in tumor cells. Biochim. Biophys. Acta 1848, 2547–2575 - PubMed
-
- Shoshan-Barmatz V., De Pinto V., Zweckstetter M., Raviv Z., Keinan N., and Arbel N. (2010) VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol. Aspects Med. 31, 227–285 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

