The Cysteine-rich Domain of the DHHC3 Palmitoyltransferase Is Palmitoylated and Contains Tightly Bound Zinc
- PMID: 26487721
- PMCID: PMC4705932
- DOI: 10.1074/jbc.M115.691147
The Cysteine-rich Domain of the DHHC3 Palmitoyltransferase Is Palmitoylated and Contains Tightly Bound Zinc
Abstract
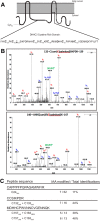
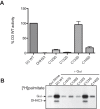
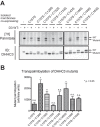
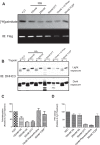
DHHC palmitoyltransferases catalyze the addition of the fatty acid palmitate to proteins on the cytoplasmic leaflet of cell membranes. There are 23 members of the highly diverse mammalian DHHC protein family, all of which contain a conserved catalytic domain called the cysteine-rich domain (CRD). DHHC proteins transfer palmitate via a two-step catalytic mechanism in which the enzyme first modifies itself with palmitate in a process termed autoacylation. The enzyme then transfers palmitate from itself onto substrate proteins. The number and location of palmitoylated cysteines in the autoacylated intermediate is unknown. In this study, we present evidence using mass spectrometry that DHHC3 is palmitoylated at the cysteine in the DHHC motif. Mutation of highly conserved CRD cysteines outside the DHHC motif resulted in activity deficits and a structural perturbation revealed by limited proteolysis. Treatment of DHHC3 with chelating agents in vitro replicated both the specific structural perturbations and activity deficits observed in conserved cysteine mutants, suggesting metal ion-binding in the CRD. Using the fluorescent indicator mag-fura-2, the metal released from DHHC3 was identified as zinc. The stoichiometry of zinc binding was measured as 2 mol of zinc/mol of DHHC3 protein. Taken together, our data demonstrate that coordination of zinc ions by cysteine residues within the CRD is required for the structural integrity of DHHC proteins.
Keywords: S-acylation; acyltransferase; fatty acylation; mass spectrometry (MS); post-translational modification (PTM); protein palmitoylation; zinc.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



Similar articles
-
Zinc co-ordination by the DHHC cysteine-rich domain of the palmitoyltransferase Swf1.Biochem J. 2013 Sep 15;454(3):427-35. doi: 10.1042/BJ20121693. Biochem J. 2013. PMID: 23790227
-
Oligomerization of DHHC protein S-acyltransferases.J Biol Chem. 2013 Aug 2;288(31):22862-70. doi: 10.1074/jbc.M113.458794. Epub 2013 Jun 22. J Biol Chem. 2013. PMID: 23793055 Free PMC article.
-
Endoplasmic reticulum localization of DHHC palmitoyltransferases mediated by lysine-based sorting signals.J Biol Chem. 2011 Nov 11;286(45):39573-84. doi: 10.1074/jbc.M111.272369. Epub 2011 Sep 18. J Biol Chem. 2011. PMID: 21926431 Free PMC article.
-
Protein Palmitoylation by DHHC Protein Family.In: Kittler JT, Moss SJ, editors. The Dynamic Synapse: Molecular Methods in Ionotropic Receptor Biology. Boca Raton (FL): CRC Press/Taylor & Francis; 2006. Chapter 5. In: Kittler JT, Moss SJ, editors. The Dynamic Synapse: Molecular Methods in Ionotropic Receptor Biology. Boca Raton (FL): CRC Press/Taylor & Francis; 2006. Chapter 5. PMID: 21204476 Free Books & Documents. Review.
-
Structure and function of DHHC protein S-acyltransferases.Biochem Soc Trans. 2017 Aug 15;45(4):923-8. doi: 10.1042/BST20160304. Epub 2017 Jun 19. Biochem Soc Trans. 2017. PMID: 28630137 Review.
Cited by
-
Substrate recruitment by zDHHC protein acyltransferases.Open Biol. 2021 Apr;11(4):210026. doi: 10.1098/rsob.210026. Epub 2021 Apr 21. Open Biol. 2021. PMID: 33878949 Free PMC article.
-
Fatty acyl recognition and transfer by an integral membrane S-acyltransferase.Science. 2018 Jan 12;359(6372):eaao6326. doi: 10.1126/science.aao6326. Science. 2018. PMID: 29326245 Free PMC article.
-
S-Palmitoylation Sorts Membrane Cargo for Anterograde Transport in the Golgi.Dev Cell. 2018 Nov 19;47(4):479-493.e7. doi: 10.1016/j.devcel.2018.10.024. Dev Cell. 2018. PMID: 30458139 Free PMC article.
-
Research progress on S-palmitoylation modification mediated by the ZDHHC family in glioblastoma.Front Cell Dev Biol. 2024 Nov 5;12:1413708. doi: 10.3389/fcell.2024.1413708. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39563863 Free PMC article. Review.
-
Crosstalk of Synapsin1 palmitoylation and phosphorylation controls the dynamicity of synaptic vesicles in neurons.Cell Death Dis. 2022 Sep 12;13(9):786. doi: 10.1038/s41419-022-05235-4. Cell Death Dis. 2022. PMID: 36097267 Free PMC article.
References
-
- Sanders S. S., Martin D. D., Butland S. L., Lavallée-Adam M., Calzolari D., Kay C., Yates J. R. 3rd, and Hayden M. R. (2015) Curation of the mammalian palmitoylome indicates a pivotal role for palmitoylation in diseases and disorders of the nervous system and cancers. PLoS Comput. Biol. 11, e1004405. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

